Use of dried blood spots to define antibody response to the Strongyloides stercoralis recombinant antigen NIE
- PMID: 25051188
- PMCID: PMC4530980
- DOI: 10.1016/j.actatropica.2014.07.007
Use of dried blood spots to define antibody response to the Strongyloides stercoralis recombinant antigen NIE
Abstract
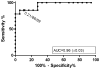
An approach to improve the diagnosis of Strongyloides stercoralis infection is the use of serologic assays utilising the NIE antigen from S. stercoralis, with good diagnostic sensitivity and excellent specificity reported. Detection of antibody eluted from dried blood spots (DBS) has shown utility in large-scale seroepidemiological studies for a range of conditions and is appealing for use with children where sample collection is difficult. We adapted an existing NIE-enzyme linked immunosorbent assay (ELISA) for the testing of strongyloides antibody response on DBS, and evaluated it in a population screening and mass drug administration programme (MDA) for strongyloidiasis conducted in an Australian indigenous community. Study participants were treated with 200 μg/kg ivermectin (>15 kg) or 3× 400 mg albendazole (<15kg). The sensitivity of the NIE DBS-ELISA was determined by receiver operator characteristic (ROC) analysis to be 85.7%. A total of 214 DBS were collected from 184 participants across two screening and MDA encounters. A total of 27 of 164 participants (16.5%) tested positive for S. stercoralis NIE-DBS prior to MDA treatment, and 6 of 50 participants (12.0%) tested positive after treatment. These prevalence values are similar to those documented by standard serology in the same community. For 30 participants where a DBS was collected at both MDA 1 and 2, a significant decline in ELISA values was evident post treatment (0.12-0.02, p=0.0012). These results are in agreement with previous studies documenting the high seroprevalence of S. stercoralis in remote Australian Indigenous communities, and suggest that collection of dried blood spots may be a useful approach for field diagnosis of S. stercoralis seroprevalence.
Keywords: Helminth infection; Indigenous health; Serological diagnosis; Strongyloides stercoralis.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures


References
-
- Biggs BA, Caruana S, Mihrshahi S, Jolley D, Leydon J, Chea L, Nuon S. Management of chronic strongyloidiasis in immigrants and refugees: is serologic testing useful? Am J Trop Med Hyg. 2009;80:788–791. - PubMed
-
- Boscolo M, Gobbo M, Mantovani W, Degani M, Anselmi M, Monteiro GB, Marocco S, Angheben A, Mistretta M, Santacatterina M, Tais S, Bisoffi Z. Evaluation of an indirect immunofluorescence assay for strongyloidiasis as a tool for diagnosis and follow-up. Clin Vaccine Immunol. 2007;14:129–133. - PMC - PubMed
-
- Carroll SM, Karthigasu KT, Grove DI. Serodiagnosis of human strongyloidiasis by an enzyme-linked immunosorbent assay. Trans R Soc Trop Med Hyg. 1981;75:706–709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

