In vitro characterization of patches of human mesenchymal stromal cells
- PMID: 25051249
- PMCID: PMC4333256
- DOI: 10.1089/ten.TEA.2013.0615
In vitro characterization of patches of human mesenchymal stromal cells
Abstract
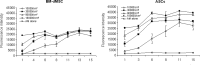
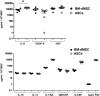
Stem cells may represent an excellent strategy to improve the healing of skin ulcers. Today the administration mode of stem cells to skin defects remains unsatisfactory. Delivering stem cells with topical treatments represents a new strategy and answering the patients' need. Mesenchymal stromal cells (MSC) have been shown to improve wound healing of cutaneous lesions and amniotic membrane (AM) is known to represent a natural scaffold for cells. The aim of this study is to develop a tissue-engineered product combining MSC and AM for clinical use. In this work we investigated whether the stromal matrix of intact human AM could constitute a scaffold for human MSC derived from either bone marrow (BM) or adipose tissue (AT). For this purpose, clinical-grade AM, MSC, and culture medium were used. We performed experiments of short-term adherence and proliferation for 15 days after the seeding of the cells. Morphological aspects and secretion profiles of MSC onto AM were studied, respectively, by scanning electron microscopy and Luminex analysis. Results demonstrated that the stromal matrix allow the adherence in much greater amount of MSC from BM or AT compared to 2D material. Experiments of proliferation showed that both kinds of MSC could proliferate on the stromal matrix and remain viable 15 days after the seeding of the cells. The 3D analysis of MSC culture demonstrated that both types of MSC invaded the stromal matrix and grew in multiple layers while retaining their fibroblastic morphology. By studying the secretion profile of MSC onto the stromal matrix, we found that both kinds of MSC secrete important cytokines and growth factors for wound healing of cutaneous lesions, such as vascular endothelial growth factor, hepatocyte growth factor, and basic fibroblast growth factor. In conclusion, these results suggest that the stromal matrix of AM seeded with MSC represents a bioactive scaffold that should be evaluated in patients with a nonhealing cutaneous wound.
Figures




References
-
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. . Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 - PubMed
-
- Delorme B., Ringe J., Gallay N., Le Vern Y., Kerboeuf D., Jorgensen C., et al. . Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood 111,2631, 2008 - PubMed
-
- Mitchell J.B., McIntosh K., Zvonic S., Garrett S., Floyd Z.E., Kloster A., et al. . Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24,376, 2006 - PubMed
-
- Strioga M., Viswanathan S., Darinskas A., Slaby O., and Michalek J.Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 21,2724, 2012 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

