δ-Opioid receptors up-regulate excitatory amino acid transporters in mouse astrocytes
- PMID: 25052197
- PMCID: PMC4294049
- DOI: 10.1111/bph.12857
δ-Opioid receptors up-regulate excitatory amino acid transporters in mouse astrocytes
Abstract
Background and purpose: Astrocytic excitatory amino acid transporters (EAATs) regulate extracellular glutamate concentrations and play a role in preventing neuroexcitotoxicity. As the δ-opioid receptor (DOP receptor) is neuroprotective against excitotoxic injury, we determined whether DOP receptor activation up-regulates EAAT expression and function.
Experimental approach: We measured mRNA and protein expression of EAAT1, EAAT2 and EAAT3 in cultured mouse astrocytes exposed to a specific DOP receptor agonist (UFP-512) with or without a DOP receptor antagonist, DOP receptor siRNA or inhibitors of PKC, PKA, PI3K, p38, MAPK, MEK and ERK, and evaluated the function of EAATs by measuring glutamate uptake.
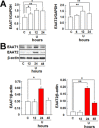
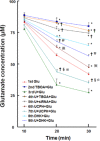
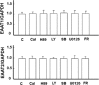
Key results: Astrocytic DOP receptor mRNA and protein were suppressed by DOP receptor siRNA knockdown. DOP receptor activation increased mRNA and protein expression of EAAT1 and EAAT2, but not EAAT3, thereby enhancing glutamate uptake of astrocytes. DOP receptor-induced EAAT1 and EAAT2 expression was largely reversed by DOP receptor antagonist naltrindole or by DOP receptor siRNA knockdown, and suppressed by inhibitors of MEK, ERK and p38. DOP receptor-accelerated glutamate uptake was inhibited by EAAT blockers, DOP receptor siRNA knockdown or inhibitors of MEK, ERK or p38. In contrast, inhibitors of PKA, PKC or PI3K had no significant effect on DOP receptor-induced EAAT expression.
Conclusions and implications: DOP receptor activation up-regulates astrocytic EAATs via MEK-ERK-p38 signalling, suggesting a critical role for DOP receptors in the regulation of astrocytic EAATs and protection against neuroexcitotoxicity. As decreased EAAT expression contributes to pathophysiology in many neurological diseases, including amyotrophic lateral sclerosis, our findings present a new platform for potential treatments of these diseases.
© 2014 The British Pharmacological Society.
Figures











References
-
- Abe K, Saito H. Possible linkage between glutamate transporter and mitogen-activated protein kinase cascade in cultured rat cortical astrocytes. J Neurochem. 2001;76:217–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

