Senescence suppressors: their practical importance in replicative lifespan extension in stem cells
- PMID: 25052377
- PMCID: PMC11113678
- DOI: 10.1007/s00018-014-1685-1
Senescence suppressors: their practical importance in replicative lifespan extension in stem cells
Abstract
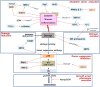
Recent animal and clinical studies report promising results for the therapeutic utilization of stem cells in regenerative medicine. Mesenchymal stem cells (MSCs), with their pluripotent nature, have advantages over embryonic stem cells in terms of their availability and feasibility. However, their proliferative activity is destined to slow by replicative senescence, and the limited proliferative potential of MSCs not only hinders the preparation of sufficient cells for in vivo application, but also draws a limitation on their potential for differentiation. This calls for the development of safe and efficient means to increase the proliferative as well as differentiation potential of MSCs. Recent advances have led to a better understanding of the underlying mechanisms and significance of cellular senescence, facilitating ways to manipulate the replicative lifespan of a variety of primary cells, including MSCs. This paper introduces a class of proteins that function as senescence suppressors. Like tumor suppressors, these proteins are lost in senescence, while their forced expression delays the onset of senescence. Moreover, treatments that increase the expression or the activity of senescence suppressors, therefore, cause expansion of the replicative and differentiation potential of MSCs. The nature of the activities and putative underlying mechanisms of the senescence suppressors will be discussed to facilitate their evaluation.
Figures
References
-
- Banfi A, Muraglia A, Dozin B, et al. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. - PubMed
-
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. - PubMed
-
- Ringdén O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. - PubMed
-
- Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. - PubMed
-
- Tokalov SV, Gruener S, Schindler S, et al. A number of bone marrow mesenchymal stem cells but neither phenotype nor differentiation capacities changes with age of rats. Mol Cells. 2007;24:255–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

