Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society
- PMID: 25053660
- PMCID: PMC4139706
- DOI: 10.1093/eurheartj/ehu274
Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society
Abstract
Aims: Homozygous familial hypercholesterolaemia (HoFH) is a rare life-threatening condition characterized by markedly elevated circulating levels of low-density lipoprotein cholesterol (LDL-C) and accelerated, premature atherosclerotic cardiovascular disease (ACVD). Given recent insights into the heterogeneity of genetic defects and clinical phenotype of HoFH, and the availability of new therapeutic options, this Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society (EAS) critically reviewed available data with the aim of providing clinical guidance for the recognition and management of HoFH.
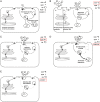
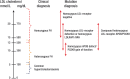
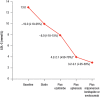
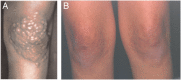
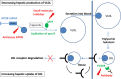
Methods and results: Early diagnosis of HoFH and prompt initiation of diet and lipid-lowering therapy are critical. Genetic testing may provide a definitive diagnosis, but if unavailable, markedly elevated LDL-C levels together with cutaneous or tendon xanthomas before 10 years, or untreated elevated LDL-C levels consistent with heterozygous FH in both parents, are suggestive of HoFH. We recommend that patients with suspected HoFH are promptly referred to specialist centres for a comprehensive ACVD evaluation and clinical management. Lifestyle intervention and maximal statin therapy are the mainstays of treatment, ideally started in the first year of life or at an initial diagnosis, often with ezetimibe and other lipid-modifying therapy. As patients rarely achieve LDL-C targets, adjunctive lipoprotein apheresis is recommended where available, preferably started by age 5 and no later than 8 years. The number of therapeutic approaches has increased following approval of lomitapide and mipomersen for HoFH. Given the severity of ACVD, we recommend regular follow-up, including Doppler echocardiographic evaluation of the heart and aorta annually, stress testing and, if available, computed tomography coronary angiography every 5 years, or less if deemed necessary.
Conclusion: This EAS Consensus Panel highlights the need for early identification of HoFH patients, prompt referral to specialized centres, and early initiation of appropriate treatment. These recommendations offer guidance for a wide spectrum of clinicians who are often the first to identify patients with suspected HoFH.
Keywords: Diagnosis; Ezetimibe; Genetics; Homozygous familial hypercholesterolaemia; Lipoprotein apheresis; Lomitapide; Mipomersen; Phenotypic heterogeneity; Statins.
© The Author 2014. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures




Comment in
-
Homozygous familial hypercholesterolemia with cutaneous xanthomas in a child.Int J Dermatol. 2022 Aug;61(8):e313-e316. doi: 10.1111/ijd.16033. Epub 2022 Jan 4. Int J Dermatol. 2022. PMID: 34982846 No abstract available.
References
-
- Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill Information Services Company; 2001. pp. 2863–2913.
-
- Kolansky DM, Cuchel M, Clark BJ, Paridon S, McCrindle BW, Wiegers SE, Araujo L, Vohra Y, Defesche JC, Wilson JM, Rader DJ. Longitudinal evaluation and assessment of cardiovascular disease in patients with homozygous familial hypercholesterolemia. Am J Cardiol. 2008;102:1438–1443. - PubMed
-
- Austin MA, Hutter CM, Zimmern RL, Humphries SE. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol. 2004;160:407–420. - PubMed
-
- Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Borén J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjærg-Hansen A European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: Consensus Statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490. - PMC - PubMed
-
- Sjouke B, Kusters DM, Kindt I, Besseling J, Defesche J, Sijbrands EJG, Roeters van Lennep JE, Stalenhoef AFH, Wiegman A, de Graaf J, Fouchier SW, Kastelein JJP, Hovingh GK. Homozygous autosomal dominant hypercholesterolemia in the Netherlands: prevalence, genotype-phenotype relationship and clinical outcome. Eur Heart J. 2014 Feb 28. Epub ahead of print. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

