Propionibacterium-produced coproporphyrin III induces Staphylococcus aureus aggregation and biofilm formation
- PMID: 25053784
- PMCID: PMC4120196
- DOI: 10.1128/mBio.01286-14
Propionibacterium-produced coproporphyrin III induces Staphylococcus aureus aggregation and biofilm formation
Abstract
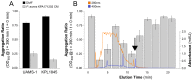
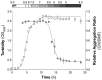
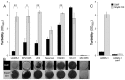
The majority of bacteria detected in the nostril microbiota of most healthy adults belong to three genera: Propionibacterium, Corynebacterium, and Staphylococcus. Among these staphylococci is the medically important bacterium Staphylococcus aureus. Almost nothing is known about interspecies interactions among bacteria in the nostrils. We observed that crude extracts of cell-free conditioned medium from Propionibacterium spp. induce S. aureus aggregation in culture. Bioassay-guided fractionation implicated coproporphyrin III (CIII), the most abundant extracellular porphyrin produced by human-associated Propionibacterium spp., as a cause of S. aureus aggregation. This aggregation response depended on the CIII dose and occurred during early stationary-phase growth, and a low pH (~4 to 6) was necessary but was not sufficient for its induction. Additionally, CIII induced plasma-independent S. aureus biofilm development on an abiotic surface in multiple S. aureus strains. In strain UAMS-1, CIII stimulation of biofilm depended on sarA, a key biofilm regulator. This study is one of the first demonstrations of a small-molecule-mediated interaction among medically relevant members of the nostril microbiota and the first description of a role for CIII in bacterial interspecies interactions. Our results indicate that CIII may be an important mediator of S. aureus aggregation and/or biofilm formation in the nostril or other sites inhabited by Propionibacterium spp. and S. aureus. Importance: Very little is known about interspecies interactions among the bacteria that inhabit the adult nostril, including Staphylococcus aureus, a potential pathogen that colonizes about a quarter of adults. We demonstrated that coproporphyrin III (CIII), a diffusible small molecule excreted by nostril- and skin-associated Propionibacterium spp., induces S. aureus aggregation in a manner dependent on dose, growth phase, and pH. CIII also induces S. aureus to form a plasma-independent surface-attached biofilm. This report is the first description of a role for CIII in bacterial interspecies interactions at any human body site and a novel demonstration that nostril microbiota physiology is influenced by small-molecule-mediated interactions.
Copyright © 2014 Wollenberg et al.
Figures



References
-
- Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, Killgore G, Tenover FC. 2006. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J. Infect. Dis. 193:172–179. 10.1086/499632 - DOI - PubMed
-
- Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, Jensen BJ, Killgore G, Tenover FC, Kuehnert MJ. 2008. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001-2004. J. Infect. Dis. 197:1226–1234. 10.1086/533494 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

