The living aortic valve: From molecules to function
- PMID: 25054122
- PMCID: PMC4104380
- DOI: 10.5339/gcsp.2014.11
The living aortic valve: From molecules to function
Abstract
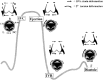
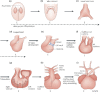
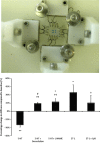
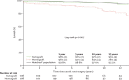
The aortic valve lies in a unique hemodynamic environment, one characterized by a range of stresses (shear stress, bending forces, loading forces and strain) that vary in intensity and direction throughout the cardiac cycle. Yet, despite its changing environment, the aortic valve opens and closes over 100,000 times a day and, in the majority of human beings, will function normally over a lifespan of 70-90 years. Until relatively recently heart valves were considered passive structures that play no active role in the functioning of a valve, or in the maintenance of its integrity and durability. However, through clinical experience and basic research the aortic valve can now be characterized as a living, dynamic organ with the capacity to adapt to its complex mechanical and biomechanical environment through active and passive communication between its constituent parts. The clinical relevance of a living valve substitute in patients requiring aortic valve replacement has been confirmed. This highlights the importance of using tissue engineering to develop heart valve substitutes containing living cells which have the ability to assume the complex functioning of the native valve.
Keywords: Cells; calcification; developmental biology; endothelium; mechanobiology; nanostructure aortic stenosis; nerves.
Figures





References
-
- El-Hamamsy I, Eryigit Z, Stevens LM, Sarang Z, George R, Clark L, Melina G, Takkenberg JJ, Yacoub MH. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet. 2010;376(9740):524–531. - PubMed
-
- Yacoub MH, Kilner PJ, Birks EJ, Misfeld M. The aortic outflow and root: a tale of dynamism and crosstalk. Ann Thorac Surg. 1999;68(3):S37–S43. - PubMed
-
- Lentink D, Müller UK, Stamhuis EJ, de Kat R, van Gestel W, Veldhuis LLM, Henningsson P, Hedenström A, Videler JJ, van Leeuwen JL. How swifts control their glide performance with morphing wings. Nature. 2007;446(7139):1082–1085. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
