Hypoxia in diabetic kidneys
- PMID: 25054148
- PMCID: PMC4094876
- DOI: 10.1155/2014/837421
Hypoxia in diabetic kidneys
Abstract
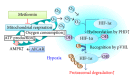
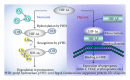
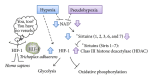
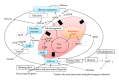
Diabetic nephropathy (DN) is now a leading cause of end-stage renal disease. In addition, DN accounts for the increased mortality in type 1 and type 2 diabetes, and then patients without DN achieve long-term survival compatible with general population. Hypoxia represents an early event in the development and progression of DN, and hypoxia-inducible factor- (HIF-) 1 mediates the metabolic responses to renal hypoxia. Diabetes induces the "fraternal twins" of hypoxia, that is, pseudohypoxia and hypoxia. The kidneys are susceptible to hyperoxia because they accept 20% of the cardiac output. Therefore, the kidneys have specific vasculature to avoid hyperoxia, that is, AV oxygen shunting. The NAD-dependent histone deacetylases (HDACs) sirtuins are seven mammalian proteins, SIRTs 1-7, which are known to modulate longevity and metabolism. Recent studies demonstrated that some isoforms of sirtuins inhibit the activation of HIF by deacetylation or noncatalyzing effects. The kidneys, which have a vascular system that protects them against hyperoxia, unfortunately experience extraordinary hypernutrition today. Then, an unexpected overload of glucose augments the oxygen consumption, which ironically results in hypoxia. This review highlights the primary role of HIF in diabetic kidneys for the metabolic adaptation to diabetes-induced hypoxia.
Figures

References
-
- Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. Journal of the American Society of Nephrology. 2006;17(1):17–25. - PubMed
-
- Haase VH. The VHL/HIF oxygen-sensing pathway and its relevance to kidney disease. Kidney International. 2006;69(8):1302–1307. - PubMed
-
- Eckardt K-U, Bernhardt W, Willam C, Wiesener M. Hypoxia-inducible transcription factors and their role in renal disease. Seminars in Nephrology. 2007;27(3):363–372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

