Membranous replication factories induced by plus-strand RNA viruses
- PMID: 25054883
- PMCID: PMC4113795
- DOI: 10.3390/v6072826
Membranous replication factories induced by plus-strand RNA viruses
Abstract
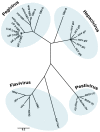
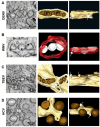
In this review, we summarize the current knowledge about the membranous replication factories of members of plus-strand (+) RNA viruses. We discuss primarily the architecture of these complex membrane rearrangements, because this topic emerged in the last few years as electron tomography has become more widely available. A general denominator is that two "morphotypes" of membrane alterations can be found that are exemplified by flaviviruses and hepaciviruses: membrane invaginations towards the lumen of the endoplasmatic reticulum (ER) and double membrane vesicles, representing extrusions also originating from the ER, respectively. We hypothesize that either morphotype might reflect common pathways and principles that are used by these viruses to form their membranous replication compartments.
Figures

References
-
- Simmonds P. The Origin of Hepatitis C Virus. In: Bartenschlager R., editor. Hepatitis C Virus: From Molecular Virology to Antiviral Therapy. 82nd ed. Volume 369 Springer Berlin Heidelberg; Berlin, Heidelberg, Germany: 2013.
-
- Lindenbach B.D., Thiel H.-J., Rice C.M. Flaviviridae: The Viruses and Their Replication. In: Fields B.N., Knipe D.M., Howley P., editors. Fields Virology. 5th ed. Raven Press; New York, NY, USA: 2001. pp. 1001–1152.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

