Resistance analyses of integrase strand transfer inhibitors within phase 3 clinical trials of treatment-naive patients
- PMID: 25054884
- PMCID: PMC4113796
- DOI: 10.3390/v6072858
Resistance analyses of integrase strand transfer inhibitors within phase 3 clinical trials of treatment-naive patients
Abstract
The integrase (IN) strand transfer inhibitors (INSTIs), raltegravir (RAL), elvitegravir (EVG) and dolutegravir (DTG), comprise the newest drug class approved for the treatment of HIV-1 infection, which joins the existing classes of reverse transcriptase, protease and binding/entry inhibitors. The efficacy of first-line regimens has attained remarkably high levels, reaching undetectable viral loads in 90% of patients by Week 48; however, there remain patients who require a change in regimen due to adverse events, virologic failure with emergent resistance or other issues of patient management. Large, randomized clinical trials conducted in antiretroviral treatment-naive individuals are required for drug approval in this population in the US, EU and other countries, with the primary endpoint for virologic success at Week 48. However, there are differences in the definition of virologic failure and the evaluation of drug resistance among the trials. This review focuses on the methodology and tabulation of resistance to INSTIs in phase 3 clinical trials of first-line regimens and discusses case studies of resistance.
Figures
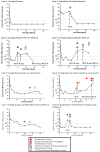
 ), the EVG Protocols blue circles (
), the EVG Protocols blue circles ( ) and the DTG Protocols orange diamonds (
) and the DTG Protocols orange diamonds ( ). Filled symbols represent the point at which resistance testing would be conducted. Open symbols represent the visit where a patient would be discontinued from study drugs. No R indicates no nucleos(t)ide reverse transcriptase inhibitor (NRTI) or INSTI resistance emerged.
). Filled symbols represent the point at which resistance testing would be conducted. Open symbols represent the visit where a patient would be discontinued from study drugs. No R indicates no nucleos(t)ide reverse transcriptase inhibitor (NRTI) or INSTI resistance emerged.References
-
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) DRAFT GUIDANCE. Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration; Silver Spring, MD, USA: 2013. Guidance for Industry Human Immunodeficiency Virus-1 Infection: Developing Antiretroviral Drugs for Treatment; pp. 1–43.
-
- European Medicines Agency (EMEA). Committee for Medicinal Products for Human Use (CHMP) London, UK: Nov 20, 2008. Guideline on the Clinical Development of Medicinal Products for the Treatment of HIV Infection. Doc Ref. EMEA/CPMP/EWP/633/02 Revision 2.
-
- British HIV Association British HIV Association guidelines for the treatment of HIV-1-positive Adults with Antiretroviral Therapy 2012. [(accessed on 17 April 2014)]. pp. 1–85. Available online: http://www.bhiva.org/TreatmentofHIV1_2012.aspx.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

