Cooperative activation of cyclobutanones and olefins leads to bridged ring systems by a catalytic [4 + 2] coupling
- PMID: 25054946
- PMCID: PMC4150356
- DOI: 10.1038/nchem.1989
Cooperative activation of cyclobutanones and olefins leads to bridged ring systems by a catalytic [4 + 2] coupling
Abstract
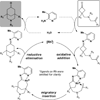
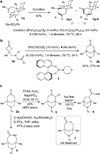
Bridged ring systems are widely found in natural products, and successful syntheses of them frequently feature intramolecular Diels-Alder reactions. These reactions are subclassified as either type I or type II depending on how the diene motif is tethered to the rest of the substrate (type I are tethered at the 1-position of the diene and type II at the 2-position). Although the type I reaction has been used with great success, the molecular scaffolds accessible by the type II reactions are limited by the strain inherent in the formation of an sp(2) carbon at a bridgehead position. Here, we describe a complementary approach that provides access to these structures through the C-C activation of cyclobutanones and their coupling with olefins. Various alkenes have been coupled with cyclobutanones to provide a range of bridged skeletons. The ketone group of the products serves as a convenient handle for downstream functionalization.
Figures

References
-
- Oppolzer W. Intramolecular [4+2] and [3+2] cycloadditions in organic synthesis. Angew. Chem. Int. Ed. 1977;16:10–23.
-
- Brieger G, Bennett JN. The intramoecular Diels-Alder reaction. Chem. Rev. 1980;80:63–97.
-
- Fallis AG. The intramolecular Diels-Alder reaction: recent advances and synthetic applications. Can. J. Chem. 1984;62:183–234.
-
- Craig D. Sterochemical aspects of the intrmolecular Diels-Alder reaction. Chem. Soc. Rev. 1987;16:187–238.
-
- Roush WR. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, Paquette LA, editors. Vol. 5. Oxford, UK: Pergamon Press; 1991. pp. 513–550.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

