Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014--United States
- PMID: 25055185
- PMCID: PMC5779422
Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014--United States
Abstract
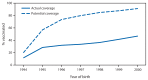
Since mid-2006, a licensed human papillomavirus (HPV) vaccine has been available and recommended by the Advisory Committee on Immunization Practices (ACIP) for routine vaccination of adolescent girls at ages 11 or 12 years. Two vaccines that protect against HPV infection are currently available in the United States. Both the quadrivalent (HPV4) and bivalent (HPV2) vaccines protect against HPV types 16 and 18, which cause 70% of cervical cancers; HPV4 also protects against HPV types 6 and 11, which cause 90% of genital warts. In 2011, the ACIP also recommended HPV4 for the routine vaccination of adolescent boys at ages 11 or 12 years. HPV vaccines can be safely co-administered with other routinely recommended vaccines, and ACIP recommends administration of all age-appropriate vaccines during a single visit. To assess progress with HPV vaccination coverage among adolescents aged 13-17 years, characterize adherence with recommendations for HPV vaccination by the 13th birthday, and describe HPV vaccine adverse reports received postlicensure, CDC analyzed data from the 2007-2013 National Immunization Survey-Teen (NIS-Teen) and national postlicensure vaccine safety data among females and males. Vaccination coverage with ≥1 dose of any HPV vaccine increased significantly from 53.8% (2012) to 57.3% (2013) among adolescent girls and from 20.8% (2012) to 34.6% (2013) among adolescent boys. Receipt of ≥1 dose of HPV among girls by age 13 years increased with each birth cohort; however, missed vaccination opportunities were common. Had HPV vaccine been administered to adolescent girls born in 2000 during health care visits when they received another vaccine, vaccination coverage for ≥1 dose by age 13 years for this cohort could have reached 91.3%. Postlicensure monitoring data continue to indicate that HPV4 is safe. Improving practice patterns so that clinicians use every opportunity to recommend HPV vaccines and address questions from parents can help realize reductions in vaccine-preventable infections and cancers caused by HPV.
Figures
References
-
- CDC. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2007;56(RR-2) - PubMed
-
- CDC. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR. 2010;59:626–9. - PubMed
-
- CDC. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR. 2011;60:1705–8. - PubMed
-
- CDC. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR. 2011;60(RR-2) - PubMed
-
- CDC, National Opinion Research Center at the University of Chicago. National Immunization Survey-Teen: a user’s guide for the 2012 public-use data file. Chicago, IL: National Opinion Research Center; 2014. Available at ftp://ftp.cdc.gov/pub/health_statistics/nchs/dataset_documentation/nis/n....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

