National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years--United States, 2013
- PMID: 25055186
- PMCID: PMC5779424
National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years--United States, 2013
Abstract
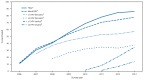
The Advisory Committee on Immunization Practices (ACIP) recommends that adolescents routinely receive 1 dose of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine, 2 doses of meningococcal conjugate (MenACWY) vaccine, and 3 doses of human papillomavirus (HPV) vaccine.* ACIP also recommends administration of "catch-up"† vaccinations, such as measles, mumps, and rubella (MMR), hepatitis B, and varicella, and, for all persons aged ≥6 months, an annual influenza vaccination. ACIP recommends administration of all age-appropriate vaccines during a single visit. To assess vaccination coverage among adolescents aged 13-17 years, CDC analyzed data from the 2013 National Immunization Survey-Teen (NIS-Teen).§ This report summarizes the results of that analysis, which show that from 2012 to 2013, coverage increased for each of the vaccines routinely recommended for adolescents: from 84.6% to 86.0% for ≥1 Tdap dose; from 74.0% to 77.8% for ≥1 MenACWY dose; from 53.8% to 57.3% for ≥1 HPV dose among females, and from 20.8% to 34.6% for ≥1 HPV dose among males. Coverage varied by state and local jurisdictions and by U.S. Department of Health and Human Services (HHS) region. Healthy People 2020 vaccination targets for adolescents aged 13-15 years were reached in 42 states for ≥1 Tdap dose, 18 for ≥1 MenACWY dose, and 11 for ≥2 varicella doses. No state met the target for ≥3 HPV doses.¶ Use of patient reminder and recall systems, immunization information systems, coverage assessment and feedback to clinicians, clinician reminders, standing orders, and other interventions can help make use of every health care visit to ensure that adolescents are fully protected from vaccine-preventable infections and cancers (5), especially when such interventions are coupled with clinicians' vaccination recommendations.
Figures
References
-
- CDC. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR. 2011;60:1705–8. - PubMed
-
- CDC. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2011;60(RR-2) - PubMed
-
- US Department of Health and Human Services. Healthy People 2020. Washington, DC: US Department of Health and Human Services; 2012. Available at http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.as....
-
- Community Preventive Services Task Force. Increasing appropriate vaccination. Atlanta, GA: Community Preventive Services Task Force; 2014. Available at http://www.thecommunityguide.org/vaccines/index.html.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

