The immunopathology of ANCA-associated vasculitis
- PMID: 25056155
- PMCID: PMC4118034
- DOI: 10.1007/s00281-014-0436-6
The immunopathology of ANCA-associated vasculitis
Abstract
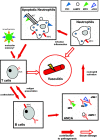
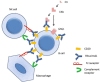
The small-vessel vasculitides are a group of disorders characterised by variable patterns of small blood vessel inflammation producing a markedly heterogeneous clinical phenotype. While any vessel in any organ may be involved, distinct but often overlapping sets of clinical features have allowed the description of three subtypes associated with the presence of circulating anti-neutrophil cytoplasmic antibodies (ANCA), namely granulomatosis with polyangiitis (GPA, formerly known as Wegener's Granulomatosis), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (eGPA, formerly known as Churg-Strauss syndrome). Together, these conditions are called the ANCA-associated vasculitidies (AAV). Both formal nomenclature and classification criteria for the syndromes have changed repeatedly since their description over 100 years ago and may conceivably do so again following recent reports showing distinct genetic associations of patients with detectable ANCA of distinct specificities. ANCA are not only useful in classifying the syndromes but substantial evidence implicates them in driving disease pathogenesis although the mechanism by which they develop and tolerance is broken remains controversial. Advances in our understanding of the pathogenesis of the syndromes have been accompanied by some progress in treatment, although much remains to be done to improve the chronic morbidity associated with the immunosuppression required for disease control.
Figures
References
-
- Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–1523. - PubMed
-
- Hagen EC, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR project for ANCA assay standardization. Kidney Int. 1998;53:743–753. - PubMed
-
- Hoffman GS. Classification of the systemic vasculitides: antineutrophil cytoplasmic antibodies, consensus and controversy. Clin Exp Rheumatol. 1998;16:111–115. - PubMed
-
- Lane SE, Watts RA, Shepstone L, Scott DG. Primary systemic vasculitis: clinical features and mortality. QJM. 2005;98:97–111. - PubMed
-
- McBride P. Photographs of a case of rapid destruction of the nose and face. 1897. J Laryngol Otol. 1991;105:1120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

