Characterisation of an aerosol exposure system to evaluate the genotoxicity of whole mainstream cigarette smoke using the in vitro γH2AX assay by high content screening
- PMID: 25056295
- PMCID: PMC4122049
- DOI: 10.1186/2050-6511-15-41
Characterisation of an aerosol exposure system to evaluate the genotoxicity of whole mainstream cigarette smoke using the in vitro γH2AX assay by high content screening
Abstract
Background: The genotoxic effect of cigarette smoke is routinely measured by treating cells with cigarette Particulate Matter (PM) at different dose levels in submerged cell cultures. However, PM exposure cannot be considered as a complete exposure as it does not contain the gas phase component of the cigarette smoke. The in vitro γH2AX assay by High Content Screening (HCS) has been suggested as a complementary tool to the standard battery of genotoxicity assays as it detects DNA double strand breaks in a high-throughput fashion. The aim of this study was to further optimise the in vitro γH2AX assay by HCS to enable aerosol exposure of human bronchial epithelial BEAS-2B cells at the air-liquid interface (ALI).
Methods: Whole mainstream cigarette smoke (WMCS) from two reference cigarettes (3R4F and M4A) were assessed for their genotoxic potential. During the study, a further characterisation of the Borgwaldt RM20S® aerosol exposure system to include single dilution assessment with a reference gas was also carried out.
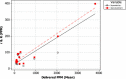
Results: The results of the optimisation showed that both reference cigarettes produced a positive genotoxic response at all dilutions tested. However, the correlation between dose and response was low for both 3R4F and M4A (Pearson coefficient, r = -0.53 and -0.44 respectively). During the additional characterisation of the exposure system, it was observed that several pre-programmed dilutions did not perform as expected.
Conclusions: Overall, the in vitro γH2AX assay by HCS could be used to evaluate WMCS in cell cultures at the ALI. Additionally, the extended characterisation of the exposure system indicates that assessing the performance of the dilutions could improve the existing routine QC checks.
Figures



References
-
- Rodgman A, Perfetti TA. In: The Chemical Components of Tobacco and Tobacco Smoke. 2. CRC Press, editor. Florida (USA); 2013.
-
- Stratton K, Shetty P, Wallace R, Bondurant S. In: Clearing the smoke: Assessing the science base for tobacco harm reduction. National Academies Press, editor. Washington, DC (USA); 2001. - PubMed
-
- World Health Organization. The Scientific Basis Of Tobacco Product Regulation. WHO; 2008. technical report series; no. 951. http://www.who.int/tobacco/global_interaction/tobreg/publications/978924.... - PubMed
-
- Phillips J, Kluss B, Richter A, Massey E. Exposure of bronchial epithelial cells to whole cigarette smoke: assessment of cellular responses. ATLA. 2005;33:239–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

