Effect of extended-release dexmethylphenidate and mixed amphetamine salts on sleep: a double-blind, randomized, crossover study in youth with attention-deficit hyperactivity disorder
- PMID: 25056567
- PMCID: PMC4362706
- DOI: 10.1007/s40263-014-0181-3
Effect of extended-release dexmethylphenidate and mixed amphetamine salts on sleep: a double-blind, randomized, crossover study in youth with attention-deficit hyperactivity disorder
Abstract
Objective: We sought to determine the dose-response effects of extended-release (ER) dexmethylphenidate (d-MPH) and ER mixed amphetamine salts (MAS) on objective measures of sleep.
Methods: This was an 8-week, double-blind, placebo-controlled, randomized, two period, crossover study of youth with attention-deficit hyperactivity disorder (ADHD) as confirmed by the Kiddie Schedule for Affective Disorders for School-Age Children-Present and Lifetime version (K-SADS-PL). Children aged 10-17 years were recruited from clinical practice, colleague referrals, and flyers. Participants were randomized to initially receive either d-MPH or MAS. During each 4-week drug period, children received three dose levels (10, 20, and 25/30 mg) in ascending order, with placebo substituted for active medication in a randomized fashion during 1 week of the study. After 4 weeks, participants were switched to the alternative medication for another 4 weeks of treatment. The main outcome measure was sleep duration as measured by actigraphy. Children, parents, and researchers were blinded to drug, dose, and placebo status.
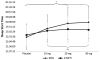
Results: Sixty-five participants met the inclusion criteria and were enrolled in the study. Of these, 37 participants with sufficient sleep data for analysis were included. Sleep schedule measures showed a significant effect for dose on sleep start time (F(1,36) = 6.284; p < 0.05), with a significantly later sleep start time when children were receiving 20- or 30-mg doses, compared with placebo (p < 0.05). A significant dose effect was found on actual sleep duration (F(1,36) = 8.112; p < 0.05), with significantly shorter actual sleep duration for subjects receiving 30 mg compared with those receiving placebo (p < 0.05). There were no significant differences on sleep duration or sleep schedule between the two stimulant medications. The trial is complete and closed to follow-up.
Conclusions: Higher stimulant doses were associated with reduced sleep duration and later sleep start times, regardless of medication class.
Trial registration: ClinicalTrials.gov: NCT00393042.
Figures


References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th American Psychiatric Association; Washington, DC: 2013.
-
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8. doi:10.1176/appi.ajp.164.6.942. - PubMed
-
- Arnsten AT. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology. CNS Drugs. 2009;23(1):33–41. doi:10.2165/00023210-200923000-00005. - PubMed
-
- Safer DJ, Zito JM, Fine EM. Increased methylphenidate usage for attention deficit disorder in the 1990s. Pediatrics. 1996;98(6):1084–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

