Catalytic enantioselective C-H functionalization of alcohols by redox-triggered carbonyl addition: borrowing hydrogen, returning carbon
- PMID: 25056771
- PMCID: PMC4150357
- DOI: 10.1002/anie.201403873
Catalytic enantioselective C-H functionalization of alcohols by redox-triggered carbonyl addition: borrowing hydrogen, returning carbon
Abstract
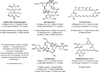
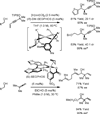
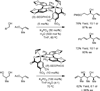
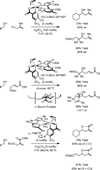
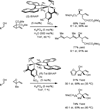
The use of alcohols and unsaturated reactants for the redox-triggered generation of nucleophile-electrophile pairs represents a broad, new approach to carbonyl addition chemistry. Discrete redox manipulations that are often required for the generation of carbonyl electrophiles and premetalated carbon-centered nucleophiles are thus avoided. Based on this concept, a broad, new family of enantioselective C-C coupling reactions that are catalyzed by iridium or ruthenium complexes have been developed, which are summarized in this Minireview.
Keywords: enantioselective catalysis; iridium; polyketide natural products; ruthenium; transfer hydrogenation.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

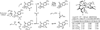




References
-
-
“The ideal synthesis creates a complex skeleton… in a sequence only of successive construction reactions involving no intermediary refunctionalizations, and leading directly to the structure of the target, not only its skeleton but also its correctly placed functionality” Hendrickson JB. J. Am. Chem. Soc. 1975;97:5784–5800.
-
-
-
For a review of redox-economy, see: Burns NZ, Baran PS, Hoffmann RW. Angew. Chem. 2009;121:2896–2910. Angew. Chem. Int. Ed.2009, 48, 2854–2867.
-
-
-
For selected reviews on selectivity and efficiency in chemical synthesis, see: Trost BM. Science. 1983;219:245–250. Newhouse T, Baran PS, Hoffmann RW. Chem. Soc. Rev. 2009;38:3010–3021.
-
-
-
For selected reviews on protecting group-free organic synthesis, see: Hoffmann RW. Synthesis. 2006:3531–3541. Young IS, Baran PS. Nature Chem. 2009;1:193–205. Roulland E. Angew. Chem. 2011;123:1260–1262. Angew. Chem. Int. Ed.2011, 50, 1226–1227.
-
-
-
Preactivation in organic synthesis, see: Han SB, Kim IS, Krische MJ. Chem. Commun. 2009:7278–7287.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

