Neuronal STIMulation at rest
- PMID: 25056876
- PMCID: PMC4150084
- DOI: 10.1126/scisignal.2005556
Neuronal STIMulation at rest
Abstract
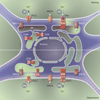
Almost a decade has passed since first STIM, and later Orai, proteins were identified as the molecular constituents of store-operated calcium entry (SOCE). Their roles in immune function have been intensely investigated, but the roles of STIM and Orai in neuronal cells have been much less clear. Lalonde et al. show that when neurons are hyperpolarized or "at rest," constitutive endoplasmic reticulum (ER) Ca(2+) release leads to SOCE-mediated activation of neuronal transcription factors. Precisely why ER Ca(2+) release is constitutive in neurons remains an important question. Irrespective of the answer, this observation provides an intriguing new perspective on why a relatively low-abundance, small-conductance channel such as Orai1 would be important in neurons, which contain a relative abundance of voltage-operated Ca(2+) channels.
Copyright © 2014, American Association for the Advancement of Science.
Figures
References
-
- Hartmann J, Karl RM, Alexander RP, Adelsberger H, Brill MS, Ruhlmann C, Ansel A, Sakimura K, Baba Y, Kurosaki T, Misgeld T, Konnerth A. STIM1 Controls Neuronal Ca(2+) Signaling, mGluR1-Dependent Synaptic Transmission, and Cerebellar Motor Behavior. Neuron. 2014;82:635–644. - PubMed
-
- Berna-Erro A, Braun A, Kraft R, Kleinschnitz C, Schuhmann MK, Stegner D, Wultsch T, Eilers J, Meuth SG, Stoll G, Nieswandt B. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal. 2009;2:ra67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

