Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection
- PMID: 25056892
- PMCID: PMC4178831
- DOI: 10.1128/JVI.01505-14
Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection
Abstract
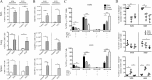
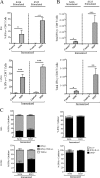
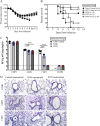
Severe acute respiratory syndrome coronavirus (SARS-CoV) caused an acute human respiratory illness with high morbidity and mortality in 2002-2003. Several studies have demonstrated the role of neutralizing antibodies induced by the spike (S) glycoprotein in protecting susceptible hosts from lethal infection. However, the anti-SARS-CoV antibody response is short-lived in patients who have recovered from SARS, making it critical to develop additional vaccine strategies. SARS-CoV-specific memory CD8 T cells persisted for up to 6 years after SARS-CoV infection, a time at which memory B cells and antivirus antibodies were undetectable in individuals who had recovered from SARS. In this study, we assessed the ability of virus-specific memory CD8 T cells to mediate protection against infection in the absence of SARS-CoV-specific memory CD4 T or B cells. We demonstrate that memory CD8 T cells specific for a single immunodominant epitope (S436 or S525) substantially protected 8- to 10-month-old mice from lethal SARS-CoV infection. Intravenous immunization with peptide-loaded dendritic cells (DCs) followed by intranasal boosting with recombinant vaccinia virus (rVV) encoding S436 or S525 resulted in accumulation of virus-specific memory CD8 T cells in bronchoalveolar lavage fluid (BAL), lungs, and spleen. Upon challenge with a lethal dose of SARS-CoV, virus-specific memory CD8 T cells efficiently produced multiple effector cytokines (gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α], and interleukin 2 [IL-2]) and cytolytic molecules (granzyme B) and reduced lung viral loads. Overall, our results show that SARS-CoV-specific memory CD8 T cells protect susceptible hosts from lethal SARS-CoV infection, but they also suggest that SARS-CoV-specific CD4 T cell and antibody responses are necessary for complete protection.
Importance: Virus-specific CD8 T cells are required for pathogen clearance following primary SARS-CoV infection. However, the role of SARS-CoV-specific memory CD8 T cells in mediating protection after SARS-CoV challenge has not been previously investigated. In this study, using a prime-boost immunization approach, we showed that virus-specific CD8 T cells protect susceptible 8- to 10-month-old mice from lethal SARS-CoV challenge. Thus, future vaccines against emerging coronaviruses should emphasize the generation of a memory CD8 T cell response for optimal protection.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, Laman JD, de Jong T, van Doornum G, Lim W, Ling AE, Chan PK, Tam JS, Zambon MC, Gopal R, Drosten C, van der Werf S, Escriou N, Manuguerra JC, Stohr K, Peiris JS, Osterhaus AD. 2003. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 362:263–270. 10.1016/S0140-6736(03)13967-0 - DOI - PMC - PubMed
-
- World Health Organization. 2014. Middle East respiratory syndrome coronavirus (MERS-CoV)—update. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/don/2014_03_25/en/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

