Characterization of the mode of action of a potent dengue virus capsid inhibitor
- PMID: 25056895
- PMCID: PMC4178822
- DOI: 10.1128/JVI.01745-14
Characterization of the mode of action of a potent dengue virus capsid inhibitor
Abstract
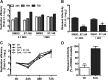
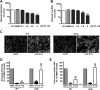
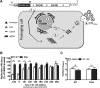
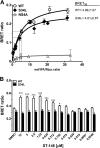
Dengue viruses (DV) represent a significant global health burden, with up to 400 million infections every year and around 500,000 infected individuals developing life-threatening disease. In spite of attempts to develop vaccine candidates and antiviral drugs, there is a lack of approved therapeutics for the treatment of DV infection. We have previously reported the identification of ST-148, a small-molecule inhibitor exhibiting broad and potent antiviral activity against DV in vitro and in vivo (C. M. Byrd et al., Antimicrob. Agents Chemother. 57:15-25, 2013, doi:10 .1128/AAC.01429-12). In the present study, we investigated the mode of action of this promising compound by using a combination of biochemical, virological, and imaging-based techniques. We confirmed that ST-148 targets the capsid protein and obtained evidence of bimodal antiviral activity affecting both assembly/release and entry of infectious DV particles. Importantly, by using a robust bioluminescence resonance energy transfer-based assay, we observed an ST-148-dependent increase of capsid self-interaction. These results were corroborated by molecular modeling studies that also revealed a plausible model for compound binding to capsid protein and inhibition by a distinct resistance mutation. These results suggest that ST-148-enhanced capsid protein self-interaction perturbs assembly and disassembly of DV nucleocapsids, probably by inducing structural rigidity. Thus, as previously reported for other enveloped viruses, stabilization of capsid protein structure is an attractive therapeutic concept that also is applicable to flaviviruses.
Importance: Dengue viruses are arthropod-borne viruses representing a significant global health burden. They infect up to 400 million people and are endemic to subtropical and tropical areas of the world. Currently, there are neither vaccines nor approved therapeutics for the prophylaxis or treatment of DV infections, respectively. This study reports the characterization of the mode of action of ST-148, a small-molecule capsid inhibitor with potent antiviral activity against all DV serotypes. Our results demonstrate that ST-148 stabilizes capsid protein self-interaction, thereby likely perturbing assembly and disassembly of viral nucleocapsids by inducing structural rigidity. This, in turn, might interfere with the release of viral RNA from incoming nucleocapsids (uncoating) as well as assembly of progeny virus particles. As previously reported for other enveloped viruses, we propose the capsid as a novel tractable target for flavivirus inhibitors.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. 10.1038/nature12060 - DOI - PMC - PubMed
-
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. 2012. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380:1559–1567. 10.1016/S0140-6736(12)61428-7 - DOI - PubMed
-
- Nowak T, Farber PM, Wengler G, Wengler G. 1989. Analyses of the terminal sequences of West Nile virus structural proteins and of the in vitro translation of these proteins allow the proposal of a complete scheme of the proteolytic cleavages involved in their synthesis. Virology 169:365–376. 10.1016/0042-6822(89)90162-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

