Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism
- PMID: 25056899
- PMCID: PMC4178784
- DOI: 10.1128/JVI.01712-14
Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism
Abstract
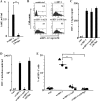
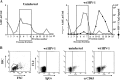
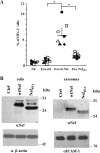
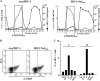
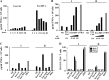
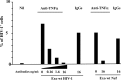
Resting CD4+ T lymphocytes resist human immunodeficiency virus (HIV) infection. Here, we provide evidence that exosomes from HIV-1-infected cells render resting human primary CD4+ T lymphocytes permissive to HIV-1 replication. These results were obtained with transwell cocultures of HIV-1-infected cells with quiescent CD4+ T lymphocytes in the presence of inhibitors of exosome release and were confirmed using exosomes purified from supernatants of HIV-1-infected primary CD4+ T lymphocytes. We found that the expression of HIV-1 Nef in exosome-producing cells is both necessary and sufficient for cell activation as well as HIV-1 replication in target CD4+ T lymphocytes. We also identified a Nef domain important for the effects we observed, i.e., the 62EEEE65 acidic cluster domain. In addition, we observed that ADAM17, i.e., a disintegrin and metalloprotease converting pro-tumor necrosis factor alpha (TNF-α) in its mature form, associates with exosomes from HIV-1-infected cells, and plays a key role in the HIV-1 replication in quiescent CD4+ T lymphocytes. Treatment with an inhibitor of ADAM17 abolished both activation and HIV-1 replication in resting CD4+ T lymphocytes. TNF-α is the downstream effector of ADAM17 since the treatment of resting lymphocytes with anti-TNF-α antibodies blocked the HIV-1 replication. The data presented here are consistent with a model where Nef induces intercellular communication through exosomes to activate bystander quiescent CD4+ T lymphocytes, thus stimulating viral spread.
Importance: Overall, our findings support the idea that HIV evolved to usurp the exosome-based intercellular communication network to favor its spread in infected hosts.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. 2008. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10:1470–1476. 10.1038/ncb1800 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

