Paradigms of sulfotransferase catalysis: the mechanism of SULT2A1
- PMID: 25056952
- PMCID: PMC4176245
- DOI: 10.1074/jbc.M114.573501
Paradigms of sulfotransferase catalysis: the mechanism of SULT2A1
Abstract
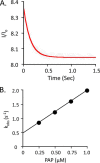
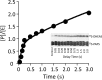
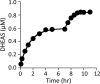
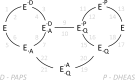
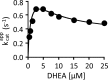
Human cytosolic sulfotransferases (SULTs) regulate the activities of thousands of signaling small molecules via transfer of the sulfuryl moiety (-SO3) from 3'-phosphoadenosine 5'-phosphosulfate (PAPS) to the hydroxyls and primary amines of acceptors. Sulfonation controls the affinities of ligands for their targets, and thereby regulates numerous receptors, which, in turn, regulate complex cellular responses. Despite their biological and medical relevance, basic SULT mechanism issues remain unresolved. To settle these issues, and to create an in-depth model of SULT catalysis, the complete kinetic mechanism of a representative member of the human SULT family, SULT2A1, was determined. The mechanism is composed of eight enzyme forms that interconvert via 22 rate constants, each of which was determined independently. The result is a complete quantitative description of the mechanism that accurately predicts complex enzymatic behavior. This is the first description of a SULT mechanism at this resolution, and it reveals numerous principles of SULT catalysis and resolves previously ambiguous issues. The structures and catalytic behaviors SULTs are highly conserved; hence, the mechanism presented here should prove paradigmatic for the family.
Keywords: DHEA; Enzyme Mechanism; Ligand-binding protein; Metabolic Regulation; Metabolism; Pre-steady State; Rate Constant; SULT2A1; Substrate Inhibition; Sulfotransferase.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Leyte A., van Schijndel H. B., Niehrs C., Huttner W. B., Verbeet M. P., Mertens K., van Mourik J. A. (1991) Sulfation of Tyr1680 of human blood coagulation factor VIII is essential for the interaction of factor VIII with von Willebrand factor. J. Biol. Chem. 266, 740–746 - PubMed
-
- Kotov A., Falany J. L., Wang J., Falany C. N. (1999) Regulation of estrogen activity by sulfation in human Ishikawa endometrial adenocarcinoma cells. J. Steroid Biochem. Mol. Biol. 68, 137–144 - PubMed
-
- Tuddenham E. G., Cooper D. N., Gitschier J., Higuchi M., Hoyer L. W., Yoshioka A., Peake I. R., Schwaab R., Olek K., Kazazian H. H., et al. (1991) Haemophilia A: database of nucleotide substitutions, deletions, insertions and rearrangements of the factor VIII gene. Nucleic Acids Res. 19, 4821–4833 - PMC - PubMed
-
- Parker C. R. (1999) Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids 64, 640–647 - PubMed
-
- Visser T. J. (1994) Role of sulfation in thyroid hormone metabolism. Chem. Biol. Interact. 92, 293–303 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

