Patient and public involvement in the early stages of clinical trial development: a systematic cohort investigation
- PMID: 25056972
- PMCID: PMC4120322
- DOI: 10.1136/bmjopen-2014-005234
Patient and public involvement in the early stages of clinical trial development: a systematic cohort investigation
Abstract
Background: Randomised controlled trials (RCTs) are considered particularly likely to benefit from patient and public involvement (PPI). Decisions made by professional researchers at the outset may go on to have a significant impact on the potential for PPI contributions.
Objective: To increase knowledge of PPI within the early development of RCTs by systematically describing the reported level, nature and acceptability of proposed PPI to the funders.
Methods: Documentation from the outline application process for all RCTs that received funding from the Health Technology Assessment (HTA) Programme 2006-2010 was requested. For each application, data were extracted on trial characteristics, references to PPI in the development of the outline application and funding Board feedback, and plans for PPI in the full application and after the trial was funded.
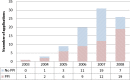
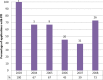
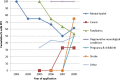
Results: 110 applications were eligible with outline applications available for 90 (82%). The cohort covered a wide range of interventions and conditions. 54% (49/90) provided some information about PPI. 26 (28.9%) indicated PPI within the development of the outline application itself; 32 (35.6%) planned involvement in the full application and 43 (48%) once the trial was funded. Recruitment at diagnosis and surgical interventions were less likely to describe PPI. Blinded trials and trials in which participants may receive placebo only, more frequently described PPI activity. The HTA commissioning Board feedback rarely referred to PPI.
Conclusions: Incorporation of PPI within the development of the outline application or specification of plans for future involvement was low. Funder requests for applicants to provide information on PPI and justification for its absence should be welcomed but further research is needed to identify the impact of this on its contributions to research. Comments on PPI by reviewers should be directional rather than state that an increase is required. Challenges facing applicants in initiating PPI prior to funding need to be addressed.
Keywords: Consumer participation; HEALTH SERVICES ADMINISTRATION & MANAGEMENT; STATISTICS & RESEARCH METHODS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- Staley K. Exploring impact: public involvement in NHS, public health and social care research. Eastleigh: INVOLVE, 2009
-
- Boote J, Baird W, Sutton A. Public involvement in the design and conduct of clinical trials: a review. Int J Interdiscip Soc Sci 2011;5:91–111
-
- Department of Health. Best research for best health: a new national health research strategy. London: Department of Health, 2006
-
- Robinson L, Newton J, Dawson P. Professionals and the public: power or partnership in health research? J Eval Clin Pract 2010;18:276–82 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
