Molecular dissection of the mechanism by which EWS/FLI expression compromises actin cytoskeletal integrity and cell adhesion in Ewing sarcoma
- PMID: 25057021
- PMCID: PMC4161506
- DOI: 10.1091/mbc.E14-01-0007
Molecular dissection of the mechanism by which EWS/FLI expression compromises actin cytoskeletal integrity and cell adhesion in Ewing sarcoma
Abstract
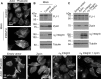
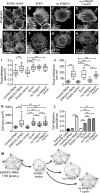
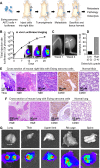
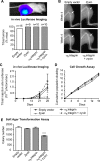
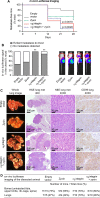
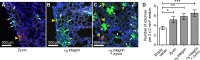
Ewing sarcoma is the second-most-common bone cancer in children. Driven by an oncogenic chromosomal translocation that results in the expression of an aberrant transcription factor, EWS/FLI, the disease is typically aggressive and micrometastatic upon presentation. Silencing of EWS/FLI in patient-derived tumor cells results in the altered expression of hundreds to thousands of genes and is accompanied by dramatic morphological changes in cytoarchitecture and adhesion. Genes encoding focal adhesion, extracellular matrix, and actin regulatory proteins are dominant targets of EWS/FLI-mediated transcriptional repression. Reexpression of genes encoding just two of these proteins, zyxin and α5 integrin, is sufficient to restore cell adhesion and actin cytoskeletal integrity comparable to what is observed when the EWS/FLI oncogene expression is compromised. Using an orthotopic xenograft model, we show that EWS/FLI-induced repression of α5 integrin and zyxin expression promotes tumor progression by supporting anchorage-independent cell growth. This selective advantage is paired with a tradeoff in which metastatic lung colonization is compromised.
© 2014 Chaturvedi, Hoffman, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
References
-
- Ambros IM, Ambros PF, Strehl S, Kovar H, Gadner H, Salzer-Kuntschik M. MIC2 is a specific marker for Ewing's sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing's sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 1991;67:1886–1893. - PubMed
-
- Amsellem V, Kryszke MH, Hervy M, Subra F, Athman R, Leh H, Brachet-Ducos C, Auclair C. The actin cytoskeleton-associated protein zyxin acts as a tumor suppressor in Ewing tumor cells. Exp Cell Res. 2005;304:443–456. - PubMed
-
- Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

