Developmental switch of leptin signaling in arcuate nucleus neurons
- PMID: 25057200
- PMCID: PMC4107412
- DOI: 10.1523/JNEUROSCI.0933-14.2014
Developmental switch of leptin signaling in arcuate nucleus neurons
Abstract
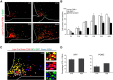
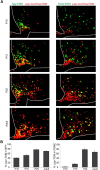
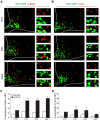
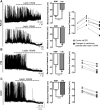
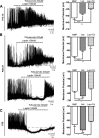
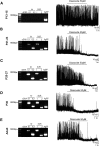
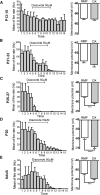
Leptin is well known for its role in the regulation of energy homeostasis in adults, a mechanism that at least partially results from the inhibition of the activity of NPY/AgRP/GABA neurons (NAG) in the arcuate nucleus of the hypothalamus (ARH). During early postnatal development in the rodent, leptin promotes axonal outgrowth from ARH neurons, and preautonomic NAG neurons are particularly responsive to leptin's trophic effects. To begin to understand how leptin could simultaneously promote axonal outgrowth from and inhibit the activity of NAG neurons, we characterized the electrochemical effects of leptin on NAG neurons in mice during early development. Here, we show that NAG neurons do indeed express a functional leptin receptor throughout the early postnatal period in the mouse; however, at postnatal days 13-15, leptin causes membrane depolarization in NAG neurons, rather than the expected hyperpolarization. Leptin action on NAG neurons transitions from stimulatory to inhibitory in the periweaning period, in parallel with the acquisition of functional ATP-sensitive potassium channels. These findings are consistent with the idea that leptin provides an orexigenic drive through the NAG system to help rapidly growing pups meet their energy requirements.
Keywords: KATP channels; NPY; arcuate nucleus; development; leptin; mouse.
Copyright © 2014 the authors 0270-6474/14/349982-13$15.00/0.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
