Enzymology of the nematode cuticle: A potential drug target?
- PMID: 25057463
- PMCID: PMC4095051
- DOI: 10.1016/j.ijpddr.2014.05.003
Enzymology of the nematode cuticle: A potential drug target?
Abstract
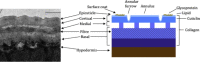
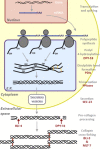
All nematodes possess an external structure known as the cuticle, which is crucial for their development and survival. This structure is composed primarily of collagen, which is secreted from the underlying hypodermal cells. Extensive studies using the free-living nematode Caenorhabditis elegans demonstrate that formation of the cuticle requires the activity of an extensive range of enzymes. Enzymes are required both pre-secretion, for synthesis of component proteins such as collagen, and post-secretion, for removal of the previous developmental stage cuticle, in a process known as moulting or exsheathment. The excretion/secretion products of numerous parasitic nematodes contain metallo-, serine and cysteine proteases, and these proteases are conserved across the nematode phylum and many are involved in the moulting/exsheathment process. This review highlights the enzymes required for cuticle formation, with a focus on the post-secretion moulting events. Where orthologues of the C. elegans enzymes have been identified in parasitic nematodes these may represent novel candidate targets for future drug/vaccine development.
Keywords: C. elegans; Collagen; Cuticle; Ecdysis; Moulting; Nematode; Protease.
Figures



References
-
- Bachinger H.P. The influence of peptidyl-prolyl cis–trans isomerase on the in vitro folding of type III collagens. J. Biol. Chem. 1987;262:17144–17148. - PubMed
-
- Bakhetia M., Charlton W., Atkinson H.J., McPherson M.J. RNA interference of dual oxidase in the plant nematode Meloidogyne incognita. Mol. Plant. Microbe Interact. 2005;18:1099–1106. - PubMed
-
- Bell A., Roberts H.C., Chappell L.H. The antiparasite effects of cyclosporin A: possible drug targets and clinical applications. Gen. Pharmacol. 1996;27:963–971. - PubMed
-
- Borchert N., Becker-Pauly C., Wagner A., Fischer P., Stocker W., Brattig N.W. Identification and characterization of onchoastacin, an astacin-like metalloproteinase from the filaria Onchocerca volvulus. Microbes Infect. 2007;9:498–506. - PubMed
-
- Brindley P.J., Gam A.A., McKerrow J.H., Neva F.A. Ss40: the zinc endopeptidase secreted by infective larvae of Strongyloides stercoralis. Exp. Parasitol. 1995;80:1–7. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

