The molecular regulation of Janus kinase (JAK) activation
- PMID: 25057888
- PMCID: PMC4112375
- DOI: 10.1042/BJ20140712
The molecular regulation of Janus kinase (JAK) activation
Abstract
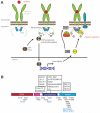
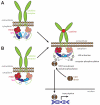
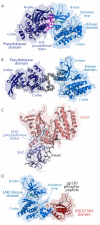
The JAK (Janus kinase) family members serve essential roles as the intracellular signalling effectors of cytokine receptors. This family, comprising JAK1, JAK2, JAK3 and TYK2 (tyrosine kinase 2), was first described more than 20 years ago, but the complexities underlying their activation, regulation and pleiotropic signalling functions are still being explored. Here, we review the current knowledge of their physiological functions and the causative role of activating and inactivating JAK mutations in human diseases, including haemopoietic malignancies, immunodeficiency and inflammatory diseases. At the molecular level, recent studies have greatly advanced our knowledge of the structures and organization of the component FERM (4.1/ezrin/radixin/moesin)-SH2 (Src homology 2), pseudokinase and kinase domains within the JAKs, the mechanism of JAK activation and, in particular, the role of the pseudokinase domain as a suppressor of the adjacent tyrosine kinase domain's catalytic activity. We also review recent advances in our understanding of the mechanisms of negative regulation exerted by the SH2 domain-containing proteins, SOCS (suppressors of cytokine signalling) proteins and LNK. These recent studies highlight the diversity of regulatory mechanisms utilized by the JAK family to maintain signalling fidelity, and suggest alternative therapeutic strategies to complement existing ATP-competitive kinase inhibitors.
Figures
References
-
- Firmbach-Kraft I, Byers M, Shows T, Dalla-Favera R, Krolewski JJ. tyk2, prototype of a novel class of non-receptor tyrosine kinase genes. Oncogene. 1990;5:1329–1336. - PubMed
-
- Rane SG, Reddy EP. JAK3: a novel JAK kinase associated with terminal differentiation of hematopoietic cells. Oncogene. 1994;9:2415–2423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

