Topical lidocaine for neuropathic pain in adults
- PMID: 25058164
- PMCID: PMC6540846
- DOI: 10.1002/14651858.CD010958.pub2
Topical lidocaine for neuropathic pain in adults
Abstract
Background: Lidocaine is a local anaesthetic that is sometimes used on the skin to treat neuropathic pain.
Objectives: To assess the analgesic efficacy of topical lidocaine for chronic neuropathic pain in adults, and to assess the associated adverse events.
Search methods: We searched CENTRAL, MEDLINE, and EMBASE from inception to 1 July 2014, together with the reference lists of retrieved papers and other reviews. We also searched ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal to identify additional published or unpublished data.
Selection criteria: We included randomised, double-blind studies of at least two weeks' duration comparing any formulation of topical lidocaine with placebo or another active treatment in chronic neuropathic pain. Participants were adults aged 18 and over. We included only full journal publication articles.
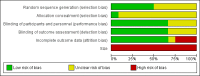
Data collection and analysis: Two review authors independently extracted efficacy and adverse event data, and examined issues of study quality. We performed analysis using three tiers of evidence. First tier evidence derived from data meeting current best standards and subject to minimal risk of bias (outcome equivalent to substantial pain intensity reduction, intention-to-treat analysis without imputation for dropouts; at least 200 participants in the comparison, 8 to 12 weeks' duration, parallel design); second tier evidence from data that failed to meet one or more of these criteria and that we considered at some risk of bias but with adequate numbers in the comparison; and third tier evidence from data involving small numbers of participants that we considered very likely to be biased or used outcomes of limited clinical utility, or both.
Main results: We included 12 studies (508 participants) in comparisons with placebo or an active control. Six studies enrolled participants with moderate or severe postherpetic neuralgia, and the remaining studies enrolled different, or mixed, neuropathic pain conditions, including trigeminal neuralgia and postsurgical or post-traumatic neuralgia. Four different formulations were used: 5% medicated patch, 5% cream, 5% gel, and 8% spray. Most studies used a cross-over design, and two used a parallel-group design. Two studies used enriched enrolment with randomised withdrawal. Seven studies used multiple doses, with one to four-week treatment periods, and five used single applications. We judged all of the studies at high risk of bias because of small size or incomplete outcome assessment, or both.There was no first or second tier evidence, and no pooling of data was possible for efficacy outcomes. Only one multiple-dose study reported our primary outcome of participants with ≥ 50% or ≥ 30% pain intensity reduction. Three single-dose studies reported participants who were pain-free at a particular time point, or had a 2-point (of 10) reduction in pain intensity. The two enriched enrolment, randomised withdrawal studies reported time to loss of efficacy. In all but one study, third tier (very low quality) evidence indicated that lidocaine was better than placebo for some measure of pain relief. Pooling multiple-dose studies across conditions demonstrated no clear evidence of an effect of lidocaine on the incidence of adverse events or withdrawals, but there were few events and the withdrawal phase of enriched enrolment designs is not suitable to assess the true impact of adverse events (very low quality evidence).
Authors' conclusions: This review found no evidence from good quality randomised controlled studies to support the use of topical lidocaine to treat neuropathic pain, although individual studies indicated that it was effective for relief of pain. Clinical experience also supports efficacy in some patients. Several large ongoing studies, of adequate duration, with clinically useful outcomes should provide more robust conclusions about both efficacy and harm.
Conflict of interest statement
JQ has no known conflicts of interest. SD, PW, and RAM have no conflicts relating to this review or any similar product.
Figures





Update of
References
References to studies included in this review
Binder 2009 {published data only}
-
- Binder A, Bruxelle J, Rogers P, Hans G, Bösl I, Baron R. Topical 5% lidocaine (lignocaine) medicated plaster treatment for post‐herpetic neuralgia: results of a double‐blind, placebo‐controlled, multinational efficacy and safety trial. Clinical Drug Investigation 2009;29(6):393‐408. [DOI: 10.2165/00044011-200929060-00003] - DOI - PubMed
Bischoff 2013 {published data only}
Cheville 2009 {published data only}
-
- Cheville AL, Sloan JA, Northfelt DW, Jillella AP, Wong GY, Bearden Iii JD, et al. Use of a lidocaine patch in the management of postsurgical neuropathic pain in patients with cancer: a phase III double‐blind crossover study (N01CB). Supportive Care in Cancer 2009;17(4):451‐60. [DOI: 10.1007/s00520-008-0542-x] - DOI - PMC - PubMed
Galer 1999 {published data only}
Galer 2002 {published data only}
-
- Galer BS, Jensen MP, Ma T, Davies PS, Rowbotham MC. The lidocaine patch 5% effectively treats all neuropathic pain qualities: results of a randomized, double‐blind, vehicle‐controlled, 3‐week efficacy study with use of the neuropathic pain scale. Clinical Journal of Pain 2002;18(5):297‐301. - PubMed
-
- Rowbotham MC, Davies PS, Galer BS. Multicenter, double‐blind, vehicle‐controlled trial of long term use of lidocaine patches for postherpetic neuralgia [abstract 184]. Abstracts of the 8th World Congress of the International Association for the Study of Pain (Vancouver, British Columbia, Canada, August 17–22). 1996:274.
Ho 2008 {published data only}
Kanai 2006 {published data only}
Kanai 2009a {published data only}
Kanai 2009b {published data only}
Meier 2003 {published data only}
Rowbotham 1995 {published data only}
References to studies excluded from this review
NCT00609323 {unpublished data only}
-
- A Randomized, Double‐Blind, Crossover Study Comparing Pliaglis™ (Lidocaine and Tetracaine) Cream 7%/7% to Placebo Cream When Applied for 60 Minutes in the Treatment of Patients With Postherpetic Neuralgia. http://clinicaltrials.gov/ct2/show/NCT00609323 (accessed 5 February 2014) 2014. [NCT: NCT00609323]
NCT01155986 {unpublished data only}
-
- Lidocaine 5% Medicated Plaster for the Topical Treatment of Localized Chronic Postoperative Neuropathic Pain. http://clinicaltrials.gov/ct2/show/NCT01155986 (accessed 5 February 2014) 2014. [NCT: NCT01155986]
Tajti 1999 {published data only}
-
- Tajti J, Szok D, Vécsei L. Topical acetylsalicylic acid versus lidocaine for postherpetic neuralgia: results of a double‐blind comparative clinical trial. Neurobiology (Bp) 1999;7(2):103‐8. - PubMed
References to studies awaiting assessment
NCT00904202 {unpublished data only}
-
- A Multicenter, Randomized, Double‐Blind, Placebo‐Controlled, Parallel‐Group Study Of Lidocaine Patch 5% Alone, Gabapentin Alone, And Lidocaine Patch 5% And Gabapentin In Combination For The Relief Of Pain In Patients With Diverse Peripheral Neuropathic Pain Conditions. http://clinicaltrials.gov/ct2/show/NCT00904202 (accessed 5 February 2014) 2014. [NCT: NCT00904202]
References to ongoing studies
EudraCT 2009‐015415‐41 {unpublished data only}
-
- Lidocaine patches in postoperative and posttraumatic neuropathic chronic skin pain ‐ A prospective, randomized, double‐blinded, placebo‐controlled, parallel, multicentre, investigator initiated study according to clinical guidelines. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:200... (accessed 5 February 2014) 2014. [Eudract number: 2009‐015415‐41]
EudraCT 2012‐000347‐28 {unpublished data only}
-
- Efficacy and safety of lidocaine 5% medicated plaster in localized chronic post‐operative neuropathic pain. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:201... (accessed 5 February 2014) 2014. [Eudract number: 2012‐000347‐28]
EudraCT 2012‐003077‐26 {unpublished data only}
-
- Topical lidocaine for the treatment of focal peripheral neuropathic pain: response in relation to pain phenotype. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:201... (accessed 5 February 2014) 2014. [Eudract number: EudraCT 2012‐003077‐26]
NCT00686127 {published data only}
-
- A Randomized, Double‐Blind, Placebo‐Controlled Trial (RDBPCT) of the Effectiveness of the Lidocaine Patch in the Management of Neuropathic Pain After Breast Cancer Surgery. ClinicalTrials.gov (accessed 5 February 2014) 2014.
NCT01752322 {published data only}
-
- Efficacy and Tolerability of Lidocaine Plaster for Treatment of Long‐term Local Nerve Pain. Clinical trials.gov (accessed 5 February 2014) 2014.
Additional references
Apkarian 2011
Bouhassira 2008
-
- Bouhassira D, Lantéri‐Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136(3):380‐7. - PubMed
Campbell 2002
-
- Campbell BJ, Rowbotham M, Davies PS, Jacob P 3rd, Benowitz NL. Systemic absorption of topical lidocaine in normal volunteers, patients with post‐herpetic neuralgia, and patients with acute herpes zoster. Journal of Pharmaceutical Sciences 2002;91(5):1343‐50. - PubMed
Chronicle 2004
Cochrane PaPaS Group 2011
-
- Cochrane Pain, Palliative and Supportive Care Group. PaPaS author and referee guidance. http://papas.cochrane.org/sites/papas.cochrane.org/files/uploads/L%20‐%2... (accessed 22 January 2013).
Derry 2012
Derry 2013
Dworkin 2008
-
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. - PubMed
Elbourne 2002
EMC 2013
-
- Electronic Medicines Compendium. Versatis 5% Medicated Plaster. Summary of Product Characteristics. www.medicines.org.uk/emc/medicine/19291 (accessed 17 December 2013).
Garnock‐Jones 2009
Geborek 2002
-
- Geborek P, Crnkic M, Petersson IF, Saxne T, South Swedish Arthritis Treatment Group. Etanercept, infliximab, and leflunomide in established rheumatoid arthritis: clinical experience using a structured follow up programme in southern Sweden. Annals of the Rheumatic Diseases 2002;61(9):793‐8. [DOI: 10.1136/ard.61.9.793] - DOI - PMC - PubMed
Gustorff 2008
-
- Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self‐reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiologica Scandinavica 2008;52(1):132‐6. - PubMed
Hall 2008
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. - PubMed
Jensen 2011
-
- Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice AS, et al. A new definition of neuropathic pain. Pain 2011;152(10):2204‐5. - PubMed
Katusic 1991
-
- Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota, 1945‐1984. Neuroepidemiology 1991;10:276‐81. - PubMed
Khan 1996
-
- Khan KS, Daya S, Jadad A. The importance of quality of primary studies in producing unbiased systematic reviews. Archives of Internal Medicine 1996;156(6):661‐6. - PubMed
Koopman 2009
-
- Koopman JS, Dieleman JP, Huygen FJ, Mos M, Martin CG, Sturkenboom MC. Incidence of facial pain in the general population. Pain 2009;147(1‐3):122‐7. - PubMed
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. - PubMed
Lunn 2009
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 978‐0192630483]
McQuay 2007
-
- McQuay HJ, Smith LA, Moore RA. Chronic pain. In: Stevens A, Raftery J, Mant J, Simpson S editor(s). Health Care Needs Assessment. 3rd Edition. Oxford: Radcliffe Publishing, 2007. [ISBN: 978‐1846190636]
Mick 2012
-
- Mick G, Correa‐Illanes G. Topical pain management with the 5% lidocaine medicated plaster ‐ a review. Current Medical Research and Opinion 2012;28(6):937‐51. - PubMed
Moher 2009
Moisset 2007
-
- Moisset X, Bouhassira D. Brain imaging of neuropathic pain. Neuroimaging 2007;37(Suppl 1):S80‐8. - PubMed
Moore 1998
-
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. - PubMed
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978–0931092695]
Moore 2009
Moore 2010a
-
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. - PubMed
Moore 2010b
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. - PMC - PubMed
Moore 2010c
-
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360‐4. - PubMed
Moore 2010d
-
- Moore RA, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers‐needed‐to‐treat analyses ‐ do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo‐controlled chronic low back pain trials. Pain 2010;151(3):592‐7. - PubMed
Moore 2011a
Moore 2011b
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. - PubMed
Moore 2011c
-
- Moore RA, Mhuircheartaigh RJ, Derry S, McQuay HJ. Mean analgesic consumption is inappropriate for testing analgesic efficacy in post‐operative pain: analysis and alternative suggestion. European Journal of Anaesthesiology 2011;28(6):427‐32. - PubMed
Moore 2012
Moore 2013a
Moore 2013b
-
- Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. - PubMed
Moore 2014a
-
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. - PubMed
Moore 2014b
O'Brien 2010
-
- O'Brien EM, Staud RM, Hassinger AD, McCulloch RC, Craggs JG, Atchison JW, et al. Patient‐centered perspective on treatment outcomes in chronic pain. Pain Medicine 2010;11(1):6‐15. - PubMed
O'Connor 2009
-
- O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. American Journal of Medicine 2009;122(10 Suppl):S22‐32. - PubMed
Rappaport 1994
-
- Rappaport ZH, Devor M. Trigeminal neuralgia: the role of self‐sustaining discharge in the trigeminal ganglion. Pain 1994;56:127‐38. - PubMed
Review Manager 2013 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013.
Sabatowski 2012
-
- Sabatowski R, Hans G, Tacken I, Kapanadze S, Buchheister B, Baron R. Safety and efficacy outcomes of long‐term treatment up to 4 years with 5% lidocaine medicated plaster in patients with post‐herpetic neuralgia. Current Medical Research and Opinion 2012;28(8):1337‐46. [DOI: 10.1185/03007995.2012.707977] - DOI - PubMed
Sawynok 2014
Snedecor 2014
Soni 2013
Straube 2008
Straube 2010
Sultan 2008
Torrance 2006
-
- Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. Journal of Pain 2006;7(4):281‐9. - PubMed
Tracey 2011
-
- Tracey I. Can neuroimaging studies identify pain endophenotypes in humans?. Nature Reviews Neurology 2011;7(3):173‐81. - PubMed
Treede 2008
-
- Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70(18):1630‐5. - PubMed
van Hecke 2014
Vos 2012
Wehrfritz 2011
-
- Wehrfritz A, Namer B, Ihmsen H, Mueller C, Filitz J, Koppert W, et al. Differential effects on sensory functions and measures of epidermal nerve fiber density after application of a lidocaine patch (5%) on healthy human skin. European Journal of Pain 2011;15(9):907‐12. [DOI: 10.1016/j.ejpain.2011.03.011] - DOI - PubMed
Wolff 2010
-
- Wolff RF, Bala MM, Westwood M, Kessels AG, Kleijnen J. 5% lidocaine medicated plaster in painful diabetic peripheral neuropathy (DPN): a systematic review. Swiss Medical Weekly 2010;140(21‐2):297‐306. [DOI: ] - PubMed
Wolff 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

