Evaluation of (89)Zr-pertuzumab in Breast cancer xenografts
- PMID: 25058168
- PMCID: PMC4224522
- DOI: 10.1021/mp500323d
Evaluation of (89)Zr-pertuzumab in Breast cancer xenografts
Abstract
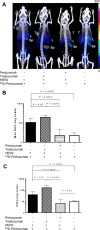
Pertuzumab is a monoclonal antibody that binds to HER2 and is used in combination with another HER2-specific monoclonal antibody, trastuzumab, for the treatment of HER2+ metastatic breast cancer. Pertuzumab binds to an HER2 binding site distinct from that of trastuzumab, and its affinity is enhanced when trastuzumab is present. We aim to exploit this enhanced affinity of pertuzumab for its HER2 binding epitope and adapt this antibody as a PET imaging agent by radiolabeling with (89)Zr to increase the sensitivity of HER2 detection in vivo. Here, we investigate the biodistribution of (89)Zr-pertuzumab in HER2-expressing BT-474 and HER2-nonexpressing MDA-MB-231 xenografts to quantitatively assess HER2 expression in vivo. In vitro cell binding studies were performed resulting in retained immunoreactivity and specificity for HER2-expressing cells. In vivo evaluation of (89)Zr-pertuzumab was conducted in severely combined immunodeficient mice, subcutaneously inoculated with BT-474 and MDA-MB-231 cells. (89)Zr-pertuzumab was systemically administered and imaged at 7 days postinjection (p.i.) followed by terminal biodistribution studies. Higher tumor uptake was observed in BT-474 compared to MDA-MB-231 xenografts with 47.5 ± 32.9 and 9.5 ± 1.7% ID/g, respectively at 7 days p.i (P = 0.0009) and blocking studies with excess unlabeled pertuzumab showed a 5-fold decrease in BT-474 tumor uptake (P = 0.0006), confirming the in vivo specificity of this radiotracer. Importantly, we observed that the tumor accumulation of (89)Zr-pertuzumab was increased in the presence of unlabeled trastuzumab, at 173 ± 74.5% ID/g (P = 0.01). Biodistribution studies correlate with PET imaging quantification using max SUV (r = 0.98, P = 0.01). Collectively, these results illustrate that (89)Zr-pertuzumab as a PET imaging agent may be beneficial for the quantitative and noninvasive assessment of HER2 expression in vivo especially for patients undergoing trastuzumab therapy.
Keywords: 89Zr; HER2; NOG; PET; breast cancer xenograft; pertuzumab; trastuzumab.
Figures



References
-
- Gaykema S. B.; Brouwers A. H.; Lub-de Hooge M. N. 89Zr-bevacizumab PET imaging in primary breast cancer. J. Nucl. Med. 2013, 54, 1014–1018. - PubMed
-
- Dijkers E. C.; Oude Munnink T. H.; Kosterink J. G.; et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2–positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. - PubMed
-
- Capelan M.; Pugliano L.; De Azambuja E. Pertuzumab: new hope for patients with HER2–positive breast cancer. Annals Oncol. 2013, 273–282. - PubMed
-
- Tamura K.; Kurihara H.; Yonemori K.; et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2–positive breast cancer. J. Nucl. Med. 2013, 54, 1869–1875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

