Metabolome analysis of Arabidopsis thaliana roots identifies a key metabolic pathway for iron acquisition
- PMID: 25058345
- PMCID: PMC4109925
- DOI: 10.1371/journal.pone.0102444
Metabolome analysis of Arabidopsis thaliana roots identifies a key metabolic pathway for iron acquisition
Abstract
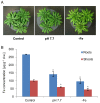
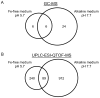
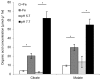
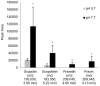
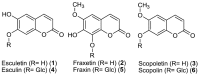
Fe deficiency compromises both human health and plant productivity. Thus, it is important to understand plant Fe acquisition strategies for the development of crop plants which are more Fe-efficient under Fe-limited conditions, such as alkaline soils, and have higher Fe density in their edible tissues. Root secretion of phenolic compounds has long been hypothesized to be a component of the reduction strategy of Fe acquisition in non-graminaceous plants. We therefore subjected roots of Arabidopsis thaliana plants grown under Fe-replete and Fe-deplete conditions to comprehensive metabolome analysis by gas chromatography-mass spectrometry and ultra-pressure liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometry. Scopoletin and other coumarins were found among the metabolites showing the strongest response to two different Fe-limited conditions, the cultivation in Fe-free medium and in medium with an alkaline pH. A coumarin biosynthesis mutant defective in ortho-hydroxylation of cinnamic acids was unable to grow on alkaline soil in the absence of Fe fertilization. Co-cultivation with wild-type plants partially rescued the Fe deficiency phenotype indicating a contribution of extracellular coumarins to Fe solubilization. Indeed, coumarins were detected in root exudates of wild-type plants. Direct infusion mass spectrometry as well as UV/vis spectroscopy indicated that coumarins are acting both as reductants of Fe(III) and as ligands of Fe(II).
Conflict of interest statement
Figures



References
-
- Thomine S, Vert G (2013) Iron transport in plants: better be safe than sorry. Curr Opin Plant Biol 16: 322–327. - PubMed
-
- Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63: 131–152. - PubMed
-
- WHO (2002) World Health Report 2002. Available: http://www.who.int/whr/2002/en/whr02_en.pdf.
-
- White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets - iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol 182: 49–84. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

