Lytic gene expression is frequent in HSV-1 latent infection and correlates with the engagement of a cell-intrinsic transcriptional response
- PMID: 25058429
- PMCID: PMC4110040
- DOI: 10.1371/journal.ppat.1004237
Lytic gene expression is frequent in HSV-1 latent infection and correlates with the engagement of a cell-intrinsic transcriptional response
Erratum in
- PLoS Pathog. 2014 Aug;10(8):e1004361
Abstract
Herpes simplex viruses (HSV) are significant human pathogens that provide one of the best-described examples of viral latency and reactivation. HSV latency occurs in sensory neurons, being characterized by the absence of virus replication and only fragmentary evidence of protein production. In mouse models, HSV latency is especially stable but the detection of some lytic gene transcription and the ongoing presence of activated immune cells in latent ganglia have been used to suggest that this state is not entirely quiescent. Alternatively, these findings can be interpreted as signs of a low, but constant level of abortive reactivation punctuating otherwise silent latency. Using single cell analysis of transcription in mouse dorsal root ganglia, we reveal that HSV-1 latency is highly dynamic in the majority of neurons. Specifically, transcription from areas of the HSV genome associated with at least one viral lytic gene occurs in nearly two thirds of latently-infected neurons and more than half of these have RNA from more than one lytic gene locus. Further, bioinformatics analyses of host transcription showed that progressive appearance of these lytic transcripts correlated with alterations in expression of cellular genes. These data show for the first time that transcription consistent with lytic gene expression is a frequent event, taking place in the majority of HSV latently-infected neurons. Furthermore, this transcription is of biological significance in that it influences host gene expression. We suggest that the maintenance of HSV latency involves an active host response to frequent viral activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
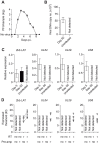

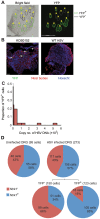
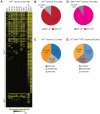

References
-
- Mester JC, Rouse BT (1991) The mouse model and understanding immunity to herpes simplex virus. Rev Infect Dis 13 Suppl 11: S935–945. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

