NLRP3 inflammasome mediates albumin-induced renal tubular injury through impaired mitochondrial function
- PMID: 25059664
- PMCID: PMC4155676
- DOI: 10.1074/jbc.M114.578260
NLRP3 inflammasome mediates albumin-induced renal tubular injury through impaired mitochondrial function
Abstract
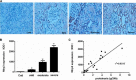
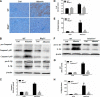
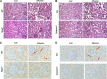
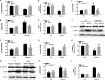
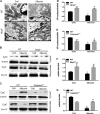
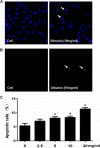
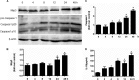
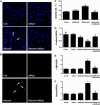
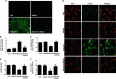
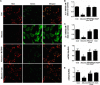
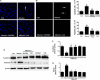
Proteinuria serves as a direct causative factor of renal tubular cell injury and is highly associated with the progression of chronic kidney disease via uncertain mechanisms. Recently, evidence demonstrated that both NLRP3 inflammasome and mitochondria are involved in the chronic kidney disease progression. The present study was undertaken to examine the role of NLRP3 inflammasome/mitochondria axis in albumin-induced renal tubular injury. In patients with proteinuria, NLRP3 was significantly up-regulated in tubular epithelial cells and was positively correlated with the severity of proteinuria. In agreement with these results, albumin remarkably activated NLRP3 inflammasome in both in vitro renal tubular cells and in vivo kidneys in parallel with significant epithelial cell phenotypic alteration and cell apoptosis. Genetic disruption of NLRP3 inflammasome remarkably attenuated albumin-induced cell apoptosis and phenotypic changes under both in vitro and in vivo conditions. In addition, albumin treatment resulted in a significant mitochondrial abnormality as evidenced by the impaired function and morphology, which was markedly reversed by invalidation of NLRP3/caspase-1 signaling pathway. Interestingly, protection of mitochondria function by Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP) or cyclosporin A (CsA) robustly attenuated albumin-induced injury in mouse proximal tubular cells. Collectively, these findings demonstrated a pathogenic role of NLRP3 inflammasome/caspase-1/mitochondria axis in mediating albumin-induced renal tubular injury. The discovery of this novel axis provides some potential targets for the treatment of proteinuria-associated renal injury.
Keywords: Apoptosis; Epithelial Cell; Inflammasome; Kidney; Mitochondria; Mitochondrial Dysfunction; NLRP3 Inflammasome; Proteinuria; Renal Tubular Cells.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Burton C., Harris K. P. (1996) The role of proteinuria in the progression of chronic renal failure. Am. J. Kidney Dis. 27, 765–775 - PubMed
-
- Eddy A. A. (2004) Proteinuria and interstitial injury. Nephrol. Dial Transplant. 19, 277–281 - PubMed
-
- Zoja C., Morigi M., Remuzzi G. (2003) Proteinuria and phenotypic change of proximal tubular cells. J. Am. Soc. Nephrol. 14, S36–S41 - PubMed
-
- Niaudet P., Rötig A. (1996) Renal involvement in mitochondrial cytopathies. Pediatr. Nephrol. 10, 368–373 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

