Host iron status and iron supplementation mediate susceptibility to erythrocytic stage Plasmodium falciparum
- PMID: 25059846
- PMCID: PMC4249681
- DOI: 10.1038/ncomms5446
Host iron status and iron supplementation mediate susceptibility to erythrocytic stage Plasmodium falciparum
Abstract
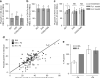
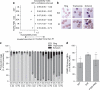
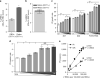
Iron deficiency and malaria have similar global distributions, and frequently co-exist in pregnant women and young children. Where both conditions are prevalent, iron supplementation is complicated by observations that iron deficiency anaemia protects against falciparum malaria, and that iron supplements increase susceptibility to clinically significant malaria, but the mechanisms remain obscure. Here, using an in vitro parasite culture system with erythrocytes from iron-deficient and replete human donors, we demonstrate that Plasmodium falciparum infects iron-deficient erythrocytes less efficiently. In addition, owing to merozoite preference for young erythrocytes, iron supplementation of iron-deficient individuals reverses the protective effects of iron deficiency. Our results provide experimental validation of field observations reporting protective effects of iron deficiency and harmful effects of iron administration on human malaria susceptibility. Because recovery from anaemia requires transient reticulocytosis, our findings imply that in malarious regions iron supplementation should be accompanied by effective measures to prevent falciparum malaria.
Figures


References
-
- Nweneka CV, Doherty CP, Cox S, Prentice A. Iron delocalisation in the pathogenesis of malarial anaemia. Trans. R. Soc. Trop. Med. Hyg. 2010;104:175–184. - PubMed
-
- Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Decreased susceptibility to Plasmodium falciparum infection in pregnant women with iron deficiency. J. Infect. Dis. 2008;198:163–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

