Ferric citrate controls phosphorus and delivers iron in patients on dialysis
- PMID: 25060056
- PMCID: PMC4310662
- DOI: 10.1681/ASN.2014020212
Ferric citrate controls phosphorus and delivers iron in patients on dialysis
Abstract
Patients on dialysis require phosphorus binders to prevent hyperphosphatemia and are iron deficient. We studied ferric citrate as a phosphorus binder and iron source. In this sequential, randomized trial, 441 subjects on dialysis were randomized to ferric citrate or active control in a 52-week active control period followed by a 4-week placebo control period, in which subjects on ferric citrate who completed the active control period were rerandomized to ferric citrate or placebo. The primary analysis compared the mean change in phosphorus between ferric citrate and placebo during the placebo control period. A sequential gatekeeping strategy controlled study-wise type 1 error for serum ferritin, transferrin saturation, and intravenous iron and erythropoietin-stimulating agent usage as prespecified secondary outcomes in the active control period. Ferric citrate controlled phosphorus compared with placebo, with a mean treatment difference of -2.2±0.2 mg/dl (mean±SEM) (P<0.001). Active control period phosphorus was similar between ferric citrate and active control, with comparable safety profiles. Subjects on ferric citrate achieved higher mean iron parameters (ferritin=899±488 ng/ml [mean±SD]; transferrin saturation=39%±17%) versus subjects on active control (ferritin=628±367 ng/ml [mean±SD]; transferrin saturation=30%±12%; P<0.001 for both). Subjects on ferric citrate received less intravenous elemental iron (median=12.95 mg/wk ferric citrate; 26.88 mg/wk active control; P<0.001) and less erythropoietin-stimulating agent (median epoetin-equivalent units per week: 5306 units/wk ferric citrate; 6951 units/wk active control; P=0.04). Hemoglobin levels were statistically higher on ferric citrate. Thus, ferric citrate is an efficacious and safe phosphate binder that increases iron stores and reduces intravenous iron and erythropoietin-stimulating agent use while maintaining hemoglobin.
Keywords: anemia; clinical trial; dialysis; phosphate binders.
Copyright © 2015 by the American Society of Nephrology.
Figures
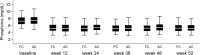

References
-
- Delmez JA, Slatopolsky E: Hyperphosphatemia: Its consequences and treatment in patients with chronic renal disease. Am J Kidney Dis 19: 303–317, 1992 - PubMed
-
- Umanath K, Blumenthal SS, Koury M, Sika M, Greco BA, Jalal DI, Reisin E, Manley J, Zeig S, Negoi DG, Hiremath AN, Lewis JB, Dwyer JP, for the Collaborative Study Group: Ferric citrate as a phosphate binder reduces IV iron and erythropoiesis stimulating agent (ESA) use. Presented at the American Society of Nephrology Kidney Week, Atlanta, GA, November 7, 2013
-
- Dwyer JP, Sika M, Schulman G, Chang IJ, Anger M, Smith M, Kaplan M, Zeig S, Koury MJ, Blumenthal SS, Lewis JB, Collaborative Study Group : Dose-response and efficacy of ferric citrate to treat hyperphosphatemia in hemodialysis patients: A short-term randomized trial. Am J Kidney Dis 61: 759–766, 2013 - PubMed
-
- Hsu CH, Patel SR, Young EW: New phosphate binding agents: Ferric compounds. J Am Soc Nephrol 10: 1274–1280, 1999 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

