A potential role for mechanical forces in the detachment of podocytes and the progression of CKD
- PMID: 25060060
- PMCID: PMC4310663
- DOI: 10.1681/ASN.2014030278
A potential role for mechanical forces in the detachment of podocytes and the progression of CKD
Abstract
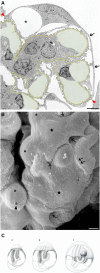
Loss of podocytes underlies progression of CKD. Detachment of podocytes from the glomerular basement membrane (GBM) rather than apoptosis or necrosis seems to be the major mechanism of podocyte loss. Such detachment of viable podocytes may be caused by increased mechanical distending and shear forces and/or impaired adhesion to the GBM. This review considers the mechanical challenges that may lead to podocyte loss by detachment from the GBM under physiologic and pathophysiologic conditions, including glomerular hypertension, hyperfiltration, hypertrophy, and outflow of filtrate from subpodocyte spaces. Furthermore, we detail the cellular mechanisms by which podocytes respond to these challenges, discuss the protective effects of angiotensin blockade, and note the questions that must be addressed to better understand the relationship between podocyte detachment and progression of CKD.
Keywords: foot process effacement; mechanical forces; podocyte detachment; progression of chronic renal failure.
Copyright © 2015 by the American Society of Nephrology.
Figures





References
-
- Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 - PubMed
-
- Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV: The podocyte’s response to stress: The enigma of foot process effacement. Am J Physiol Renal Physiol 304: F333–F347, 2013 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

