Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae
- PMID: 25060788
- PMCID: PMC4140993
- DOI: 10.1161/ATVBAHA.114.303342
Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae
Abstract
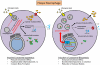
Objective: Recent reports of a proatherogenic phenotype in mice with macrophage-specific autophagy deficiency have renewed interest in the role of the autophagy-lysosomal system in atherosclerosis. Lysosomes have the unique ability to process both exogenous material, including lipids and autophagy-derived cargo such as dysfunctional proteins/organelles. We aimed to understand the effects of an atherogenic lipid environment on macrophage lysosomes and to evaluate novel ways to modulate this system.
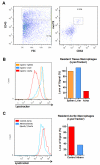
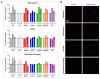
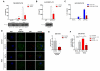
Approach and results: Using a variety of complementary techniques, we show that oxidized low-density lipoproteins and cholesterol crystals, commonly encountered lipid species in atherosclerosis, lead to profound lysosomal dysfunction in cultured macrophages. Disruptions in lysosomal pH, proteolytic capacity, membrane integrity, and morphology are readily seen. Using flow cytometry, we find that macrophages isolated from atherosclerotic plaques also display features of lysosome dysfunction. We then investigated whether enhancing lysosomal function can be beneficial. Transcription factor EB (TFEB) is the only known transcription factor that is a master regulator of lysosomal biogenesis although its role in macrophages has not been studied. Lysosomal stress induced by chloroquine or atherogenic lipids leads to TFEB nuclear translocation and activation of lysosomal and autophagy genes. TFEB overexpression in macrophages further augments this prodegradative response and rescues several deleterious effects seen with atherogenic lipid loading as evidenced by blunted lysosomal dysfunction, reduced secretion of the proinflammatory cytokine interleukin-1β, enhanced cholesterol efflux, and decreased polyubiquitinated protein aggregation.
Conclusions: Taken together, these data demonstrate that lysosomal function is markedly impaired in atherosclerosis and suggest that induction of a lysosomal biogenesis program in macrophages has antiatherogenic effects.
Keywords: atherosclerosis; autophagy; inflammasome; lipid metabolism; lysosomes; macrophages.
© 2014 American Heart Association, Inc.
Figures



References
-
- Go AS, Mozaffarian D, Roger VL, et al. Executive summary: Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:399–410. - PubMed
-
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

