Abuse potential of intranasal buprenorphine versus buprenorphine/naloxone in buprenorphine-maintained heroin users
- PMID: 25060839
- PMCID: PMC4305506
- DOI: 10.1111/adb.12163
Abuse potential of intranasal buprenorphine versus buprenorphine/naloxone in buprenorphine-maintained heroin users
Abstract
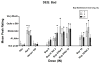
In spite of the clinical utility of buprenorphine, parenteral abuse of this medication has been reported in several laboratory investigations and in the real world. Studies have demonstrated lower abuse liability of the buprenorphine/naloxone combination relative to buprenorphine alone. However, clinical research has not yet examined the utility of the combined formulation to deter intranasal use in a buprenorphine-maintained population. Heroin-using volunteers (n = 12) lived in the hospital for 8-9 weeks and were maintained on each of three sublingual buprenorphine doses (2, 8, 24 mg). Under each maintenance dose, participants completed laboratory sessions during which the reinforcing and subjective effects of intranasal doses of buprenorphine (8, 16 mg), buprenorphine/naloxone (8/2, 8/8, 8/16, 16/4 mg) and controls (placebo, heroin 100 mg, naloxone 4 mg) were assessed. Intranasal buprenorphine alone typically produced increases in positive subjective effects and the 8 mg dose was self-administered above the level of placebo. The addition of naloxone dose dependently reduced positive subjective effects and increased aversive effects. No buprenorphine/naloxone combination dose was self-administered significantly more than placebo. These data suggest that within a buprenorphine-dependent population, intranasal buprenorphine/naloxone has reduced abuse potential in comparison to buprenorphine alone. These data strongly argue in favor of buprenorphine/naloxone rather than buprenorphine alone as the more reasonable option for managing the risk of buprenorphine misuse.
Keywords: Abuse liability; buprenorphine; intranasal; opioids; self-administration.
© 2014 Society for the Study of Addiction.
Figures

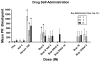
Similar articles
-
Intranasal buprenorphine alone and in combination with naloxone: Abuse liability and reinforcing efficacy in physically dependent opioid abusers.Drug Alcohol Depend. 2016 May 1;162:190-8. doi: 10.1016/j.drugalcdep.2016.03.005. Epub 2016 Mar 14. Drug Alcohol Depend. 2016. PMID: 27012435 Free PMC article. Clinical Trial.
-
Abuse liability of intravenous buprenorphine/naloxone and buprenorphine alone in buprenorphine-maintained intravenous heroin abusers.Addiction. 2010 Apr;105(4):709-18. doi: 10.1111/j.1360-0443.2009.02843.x. Addiction. 2010. PMID: 20403021 Free PMC article. Clinical Trial.
-
The pharmacodynamic and pharmacokinetic profile of intranasal crushed buprenorphine and buprenorphine/naloxone tablets in opioid abusers.Addiction. 2011 Aug;106(8):1460-73. doi: 10.1111/j.1360-0443.2011.03424.x. Epub 2011 May 3. Addiction. 2011. PMID: 21395892 Free PMC article. Clinical Trial.
-
Pharmacokinetics of the combination tablet of buprenorphine and naloxone.Drug Alcohol Depend. 2003 May 21;70(2 Suppl):S39-47. doi: 10.1016/s0376-8716(03)00058-9. Drug Alcohol Depend. 2003. PMID: 12738349 Review.
-
Buprenorphine/naloxone: a review of its use in the treatment of opioid dependence.Drugs. 2009;69(5):577-607. doi: 10.2165/00003495-200969050-00006. Drugs. 2009. PMID: 19368419 Review.
Cited by
-
Abuse liability of intravenous buprenorphine vs. buprenorphine/naloxone: Importance of absolute naloxone amount.Drug Alcohol Depend. 2017 Oct 1;179:362-369. doi: 10.1016/j.drugalcdep.2017.06.033. Epub 2017 Jul 26. Drug Alcohol Depend. 2017. PMID: 28844013 Free PMC article.
-
Buprenorphine Prescribing: To Expand or Not to Expand.J Psychiatr Pract. 2016 May;22(3):183-92. doi: 10.1097/PRA.0000000000000154. J Psychiatr Pract. 2016. PMID: 27123798 Free PMC article. Review.
-
Intranasal buprenorphine alone and in combination with naloxone: Abuse liability and reinforcing efficacy in physically dependent opioid abusers.Drug Alcohol Depend. 2016 May 1;162:190-8. doi: 10.1016/j.drugalcdep.2016.03.005. Epub 2016 Mar 14. Drug Alcohol Depend. 2016. PMID: 27012435 Free PMC article. Clinical Trial.
-
Reconsidering the usefulness of long-term high-dose buprenorphine.Front Psychiatry. 2024 Jul 23;15:1401676. doi: 10.3389/fpsyt.2024.1401676. eCollection 2024. Front Psychiatry. 2024. PMID: 39114740 Free PMC article.
-
Results from a long-term open-label extension study of adjunctive buprenorphine/samidorphan combination in patients with major depressive disorder.Neuropsychopharmacology. 2019 Dec;44(13):2268-2276. doi: 10.1038/s41386-019-0451-3. Epub 2019 Jun 29. Neuropsychopharmacology. 2019. PMID: 31254971 Free PMC article. Clinical Trial.
References
-
- Alho H, Sinclair D, Vuori E, Holopainen A. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75–78. - PubMed
-
- Auriacombe M, Fatsea M, Dubernet J, Daulouede J, Tignol J. French field experience with buprenorphine. Am J Addict. 2004;13(Suppl 1):S17–S28. - PubMed
-
- Bedi NS, Ray R, Jain R, Dhar NK. Abuse liability of buprenorphine-a study among experienced drug users. Indian J Physiol Pharmacol. 1998;42(1):95–100. - PubMed
-
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther. 1988;247:47–53. - PubMed
-
- Bullingham RE, McQuay HJ, Moore A, Bennett MR. Buprenorphine kinetics. Clin Pharmacol Ther. 1980;28(5):667–672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

