A new form of macrothrombocytopenia induced by a germ-line mutation in the PRKACG gene
- PMID: 25061177
- PMCID: PMC4199957
- DOI: 10.1182/blood-2014-01-551820
A new form of macrothrombocytopenia induced by a germ-line mutation in the PRKACG gene
Abstract
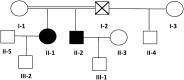
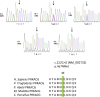
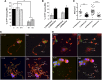
Macrothrombocytopenias are the most important subgroup of inherited thrombocytopenias. This subgroup is particularly heterogeneous because the affected genes are involved in various functions such as cell signaling, cytoskeleton organization, and gene expression. Herein we describe the clinical and hematological features of a consanguineous family with a severe autosomal recessive macrothrombocytopenia associated with a thrombocytopathy inducing a bleeding tendency in the homozygous mutated patients. Platelet activation and cytoskeleton reorganization were impaired in these homozygous patients. Exome sequencing identified a c.222C>G mutation (missense p.74Ile>Met) in PRKACG, a gene encoding the γ-catalytic subunit of the cyclic adenosine monophosphate-dependent protein kinase, the mutated allele cosegregating with the macrothrombocytopenia. We demonstrate that the p.74Ile>Met PRKACG mutation is associated with a marked defect in proplatelet formation and a low level in filamin A in megakaryocytes (MKs). The defect in proplatelet formation was rescued in vitro by lentiviral vector-mediated overexpression of wild-type PRKACG in patient MKs. We thus conclude that PRKACG is a new central actor in platelet biogenesis and a new gene involved in inherited thrombocytopenia with giant platelets associated with a thrombocytopathy.
© 2014 by The American Society of Hematology.
Figures



Comment in
-
Inherited macrothrombocytopenias on the rise.Blood. 2014 Oct 16;124(16):2473-5. doi: 10.1182/blood-2014-08-592329. Blood. 2014. PMID: 25323684
References
-
- Balduini CL, Savoia A, Seri M. Inherited thrombocytopenias frequently diagnosed in adults. J Thromb Haemost. 2013;11(6):1006–1019. - PubMed
-
- Stevenson WS, Morel-Kopp MC, Chen Q, et al. GFI1B mutation causes a bleeding disorder with abnormal platelet function. J Thromb Haemost. 2013;11(11):2039–2047. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

