Transcriptome profiling identifies ABA mediated regulatory changes towards storage filling in developing seeds of castor bean (Ricinus communis L.)
- PMID: 25061509
- PMCID: PMC4109380
- DOI: 10.1186/2045-3701-4-33
Transcriptome profiling identifies ABA mediated regulatory changes towards storage filling in developing seeds of castor bean (Ricinus communis L.)
Abstract
Background: The potential biodiesel plant castor bean (Ricinus communis) has been in the limelight for bioenergy research due to the availability of its genome which raises the bar for genome-wide studies claiming advances that impact the "genome-phenome challenge". Here we report the application of phytohormone ABA as an exogenous factor for the improvement of storage reserve accumulation with a focus on the complex interaction of pathways associated with seed filling.
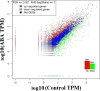
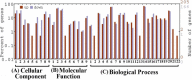
Results: After the application of exogenous ABA treatments, we measured an increased ABA levels in the developing seeds cultured in vitro using the ELISA technique and quantified the content of major biomolecules (including total lipids, sugars and protein) in treated seeds. Exogenous ABA (10 μM) enhanced the accumulation of soluble sugar content (6.3%) followed by deposition of total lipid content (4.9 %). To elucidate the possible ABA signal transduction pathways towards overall seed filling, we studied the differential gene expression analysis using Illumina RNA-Sequencing technology, resulting in 2568 (1507-up/1061-down regulated) differentially expressed genes were identified. These genes were involved in sugar metabolism (such as glucose-6-phosphate, fructose 1,6 bis-phosphate, glycerol-3-phosphate, pyruvate kinase), lipid biosynthesis (such as ACS, ACBP, GPAT2, GPAT3, FAD2, FAD3, SAD1 and DGAT1), storage proteins synthesis (such as SGP1, zinc finger protein, RING H2 protein, nodulin 55 and cytochrome P450), and ABA biosynthesis (such as NCED1, NCED3 and beta carotene). Further, we confirmed the validation of RNA-Sequencing data by Semi-quantitative RT-PCR analysis.
Conclusions: Taken together, metabolite measurements supported by genes and pathway expression results indicated in this study provide new insights to understand the ABA signaling mechanism towards seed storage filling and also contribute useful information for facilitating oilseed crop functional genomics on an aim for utilizing castor bean agricultural and bioenergy use.
Keywords: ABA signaling; Castor bean; Developing seeds; ELISA; High throughput RNA-Seq; Storage reserve.
Figures



References
-
- Zhang Y, Adams IP, Ratledge C. Malic enzyme: the controlling activity for lipid production? Overexpression of malic enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid accumulation. Microbiol. 2007;153:2013–2025. - PubMed
-
- Kanno Y, Jikumaru Y, Hanada A, Nambara E, Abrams SR, Kamiya Y, Seo M. Comprehensive hormone profiling in developing seeds of Arabidopsis seeds: Examination of the site of ABA biosynthesis, ABA transport and hormone interactions. Plant Cell Physiol. 2010;51:1988–2001. - PubMed
-
- Vahdati K, Bayat S, Ebrahimzadeh H, Jariteh M, Mirmasoumi M. Effect of exogenous ABA on somatic embryo maturation and germination in persian walnut (Juglans regia L.) Plant Cell Tissue Organ Cult. 2008;93:163–171.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

