Cloning and characterization of a norbelladine 4'-O-methyltransferase involved in the biosynthesis of the Alzheimer's drug galanthamine in Narcissus sp. aff. pseudonarcissus
- PMID: 25061748
- PMCID: PMC4111509
- DOI: 10.1371/journal.pone.0103223
Cloning and characterization of a norbelladine 4'-O-methyltransferase involved in the biosynthesis of the Alzheimer's drug galanthamine in Narcissus sp. aff. pseudonarcissus
Abstract
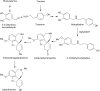
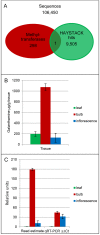
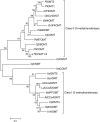
Galanthamine is an Amaryllidaceae alkaloid used to treat the symptoms of Alzheimer's disease. This compound is primarily isolated from daffodil (Narcissus spp.), snowdrop (Galanthus spp.), and summer snowflake (Leucojum aestivum). Despite its importance as a medicine, no genes involved in the biosynthetic pathway of galanthamine have been identified. This absence of genetic information on biosynthetic pathways is a limiting factor in the development of synthetic biology platforms for many important botanical medicines. The paucity of information is largely due to the limitations of traditional methods for finding biochemical pathway enzymes and genes in non-model organisms. A new bioinformatic approach using several recent technological improvements was applied to search for genes in the proposed galanthamine biosynthetic pathway, first targeting methyltransferases due to strong signature amino acid sequences in the proteins. Using Illumina sequencing, a de novo transcriptome assembly was constructed for daffodil. BLAST was used to identify sequences that contain signatures for plant O-methyltransferases in this transcriptome. The program HAYSTACK was then used to identify methyltransferases that fit a model for galanthamine biosynthesis in leaf, bulb and inflorescence tissues. One candidate gene for the methylation of norbelladine to 4'-O-methylnorbelladine in the proposed galanthamine biosynthetic pathway was identified. This methyltransferase cDNA was expressed in E. coli and the protein purified by affinity chromatography. The resulting protein was found to be a norbelladine 4'-O-methyltransferase (NpN4OMT) of the proposed galanthamine biosynthetic pathway.
Conflict of interest statement
Figures

References
-
- Gabrielsen B, Monath TP, Huggins JW, Kefauver DF, Pettit GR, et al. (1992) Antiviral (RNA) activity of selected Amaryllidaceae isoquinoline constituents and synthesis of related substances. J Nat Prod 55: 1569–1581. - PubMed
-
- Havelek R, Seifrtova M, Kralovec K, Bruckova L, Cahlikova L, et al. (2014) The effect of Amaryllidaceae alkaloids haemanthamine and haemanthidine on cell cycle progression and apoptosis in p53-negative human leukemic Jurkat cells. Phytomedicine 21: 479–490. - PubMed
-
- Liu J, Hu WX, He LF, Ye M, Li Y (2004) Effects of lycorine on HL-60 cells via arresting cell cycle and inducing apoptosis. FEBS Lett 578: 245–250. - PubMed
-
- Uyeo S, Kobayashi S (1953) Lycoris alkaloids. XXIV. Isolation and characterization of lycoremine. Pharm Bull 1: 139–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

