Lymphatic regulator PROX1 determines Schlemm's canal integrity and identity
- PMID: 25061877
- PMCID: PMC4153702
- DOI: 10.1172/JCI75392
Lymphatic regulator PROX1 determines Schlemm's canal integrity and identity
Abstract
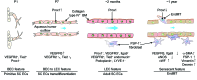
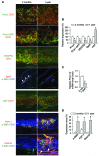
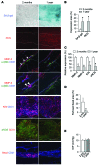
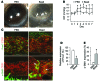
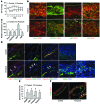
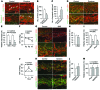
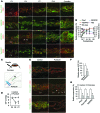
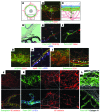
Schlemm's canal (SC) is a specialized vascular structure in the eye that functions to drain aqueous humor from the intraocular chamber into systemic circulation. Dysfunction of SC has been proposed to underlie increased aqueous humor outflow (AHO) resistance, which leads to elevated ocular pressure, a factor for glaucoma development in humans. Here, using lymphatic and blood vasculature reporter mice, we determined that SC, which originates from blood vessels during the postnatal period, acquires lymphatic identity through upregulation of prospero homeobox protein 1 (PROX1), the master regulator of lymphatic development. SC expressed lymphatic valve markers FOXC2 and integrin α9 and exhibited continuous vascular endothelial-cadherin (VE-cadherin) junctions and basement membrane, similar to collecting lymphatics. SC notably lacked luminal valves and expression of the lymphatic endothelial cell markers podoplanin and lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1). Using an ocular puncture model, we determined that reduced AHO altered the fate of SC both during development and under pathologic conditions; however, alteration of VEGF-C/VEGFR3 signaling did not modulate SC integrity and identity. Intriguingly, PROX1 expression levels linearly correlated with SC functionality. For example, PROX1 expression was reduced or undetectable under pathogenic conditions and in deteriorated SCs. Collectively, our data indicate that PROX1 is an accurate and reliable biosensor of SC integrity and identity.
Figures

Comment in
-
Schlemm's canal: more than meets the eye, lymphatics in disguise.J Clin Invest. 2014 Sep;124(9):3701-3. doi: 10.1172/JCI77507. Epub 2014 Jul 25. J Clin Invest. 2014. PMID: 25061871 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

