Cytoprotective propensity of Bacopa monniera against hydrogen peroxide induced oxidative damage in neuronal and lung epithelial cells
- PMID: 25062987
- PMCID: PMC4698260
- DOI: 10.1007/s10616-014-9767-3
Cytoprotective propensity of Bacopa monniera against hydrogen peroxide induced oxidative damage in neuronal and lung epithelial cells
Abstract
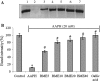
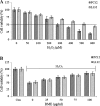
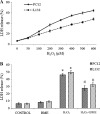
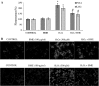
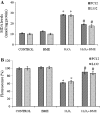
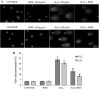
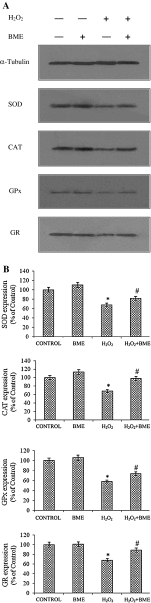
Hydrogen peroxide (H2O2), a major reactive oxygen species (ROS) produced during oxidative stress, is toxic to the cells. Hence, H2O2 has been extensively used to study the effects of antioxidant and cytoprotective role of phytochemicals. In the present investigation H2O2 was used to induce oxidative stress via ROS production within PC12 and L132 cells. Cytoprotective propensity of Bacopa monniera extract (BME) was confirmed by cell viability assays, ROS estimation, lipid peroxidation, mitochondria membrane potential assay, comet assay followed by gene expression studies of antioxidant enzymes in PC12 and L132 cells treated with H2O2 for 24 h with or without BME pre-treatment. Our results elucidate that BME possesses radical scavenging activity by scavenging 2,2-diphenyl-1-picrylhydrazyl, 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), superoxide radical, and nitric oxide radicals. The IC50 value of BME against these radicals was found to be 226.19, 15.17, 30.07, and 34.55 µg/ml, respectively). The IC50 of BME against ROS, lipid peroxidation and protein carbonylation was found to be 1296.53, 753.22, and 589.04 µg/ml in brain and 1137.08, 1079.65, and 11101.25 µg/ml in lung tissues, respectively. Further cytoprotective potency of the BME ameliorated the mitochondrial and plasma membrane damage induced by H2O2 as evidenced by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and lactate dehydrogenase leakage assays in both PC12 and L132 cells. H2O2 induced cellular, nuclear and mitochondrial membrane damage was restored by BME pre-treatment. H2O2 induced depleted antioxidant status was also replenished by BME pre-treatment. This was confirmed by spectrophotometric analysis, semi-quantitative RT-PCR and western blot studies. These results justify the traditional usage of BME based on its promising antioxidant and cytoprotective property.
Keywords: Antioxidant enzymes; Bacopa monniera; Cytotoxicity; Hydrogen peroxide; L132 cells; Oxidative stress; PC12 cells; Reactive oxygen species.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

