Melanogenesis inhibitory effect of aerial part of Pueraria thunbergiana in vitro and in vivo
- PMID: 25063049
- PMCID: PMC4282881
- DOI: 10.1007/s00403-014-1489-z
Melanogenesis inhibitory effect of aerial part of Pueraria thunbergiana in vitro and in vivo
Abstract
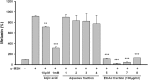
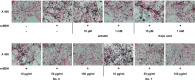
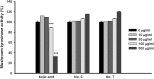
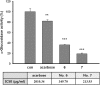
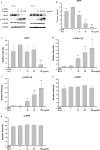
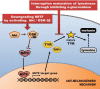
Melanin is major factor that determines skin color as well as one of the defense systems that prevent the UV-induced damage. In case of abnormal concentration of melanin, skin diseases or problems occur such as albinism, leukoplakia, melasma, freckles, moles, and lentigo. With the lifespan of humans has been extended, importance of 'life quality' has been increased. White and clean skin is very important part of the satisfaction of appearance, especially for Asia women. The aim of this study was to find an anti-melanogenesis activity for which the aerial part of Pueraria thunbergiana can be utilized based on the increase in demands for cosmetics, particularly natural products. We demonstrated anti-pigmentation effects of aerial part of P. thunbergiana by measuring melanin content and through staining in the B16F10 melanoma cell line. The aerial part of P. thunbergiana decreased tyrosinase activity significantly in B16F10 cell cultures, while there is no direct effect on enzyme in cell-free conditions. To define the mechanisms, real-time PCR, western blot, glucosidase activity and antioxidant activity assay were implemented. As results, we demonstrated that aerial part of P. thunbergiana has anti-melanogenesis activity via two mechanisms. One is downgrading microphthalmia-associated transcription factor by activating Akt/GSK-3β. Consequently, transcription of tyrosinase and tyrosinase-related protein 1 is decreased. Another is interrupting maturation of tyrosinase through inhibiting α-glucosidase. Furthermore, aerial part of P. thunbergiana showed great efficacy on pigmentation in vivo. These results suggest that aerial part of P. thunbergiana can be used as an anti-melanogenic agent.
Figures
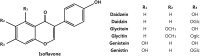
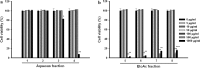






References
-
- Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim Biophys Acta. 1996;1313(2):130–138. doi: 10.1016/0167-4889(96)00063-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

