Visualisation of chicken macrophages using transgenic reporter genes: insights into the development of the avian macrophage lineage
- PMID: 25063453
- PMCID: PMC4197536
- DOI: 10.1242/dev.105593
Visualisation of chicken macrophages using transgenic reporter genes: insights into the development of the avian macrophage lineage
Abstract
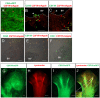
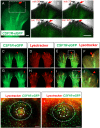
We have generated the first transgenic chickens in which reporter genes are expressed in a specific immune cell lineage, based upon control elements of the colony stimulating factor 1 receptor (CSF1R) locus. The Fms intronic regulatory element (FIRE) within CSF1R is shown to be highly conserved in amniotes and absolutely required for myeloid-restricted expression of fluorescent reporter genes. As in mammals, CSF1R-reporter genes were specifically expressed at high levels in cells of the macrophage lineage and at a much lower level in granulocytes. The cell lineage specificity of reporter gene expression was confirmed by demonstration of coincident expression with the endogenous CSF1R protein. In transgenic birds, expression of the reporter gene provided a defined marker for macrophage-lineage cells, identifying the earliest stages in the yolk sac, throughout embryonic development and in all adult tissues. The reporter genes permit detailed and dynamic visualisation of embryonic chicken macrophages. Chicken embryonic macrophages are not recruited to incisional wounds, but are able to recognise and phagocytose microbial antigens.
Keywords: Chicken; Dendritic cells; Embryonic development; Immunity; Macrophages; Transgenics.
© 2014. Published by The Company of Biologists Ltd.
Figures




References
-
- Befus A. D., Johnston N., Leslie G. A., Bienenstock J. (1980). Gut-associated lymphoid tissue in the chicken. I. Morphology, ontogeny, and some functional characteristics of Peyer's patches. J. Immunol. 125, 2626-2632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

