Noninvasive inhaled nitric oxide does not prevent bronchopulmonary dysplasia in premature newborns
- PMID: 25063725
- PMCID: PMC4464845
- DOI: 10.1016/j.jpeds.2014.06.018
Noninvasive inhaled nitric oxide does not prevent bronchopulmonary dysplasia in premature newborns
Abstract
Objective: To assess the efficacy and safety of early, noninvasive inhaled nitric oxide (iNO) therapy in premature newborns who do not require mechanical ventilation.
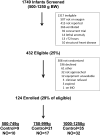
Study design: We performed a multicenter randomized trial including 124 premature newborns who required noninvasive supplemental oxygen within the first 72 hours after birth. Newborns were stratified into 3 different groups by birth weight (500-749, 750-999, 1000-1250 g) prior to randomization to iNO (10 ppm) or placebo gas (controls) until 30 weeks postmenstrual age. The primary outcome was a composite of death or bronchopulmonary dysplasia (BPD) at 36 weeks postmenstrual age. Secondary outcomes included the need for and duration of mechanical ventilation, severity of BPD, and safety outcomes.
Results: There was no difference in the incidence of death or BPD in the iNO and placebo groups (42% vs 40%, P = .86, relative risk = 1.06, 0.7-1.6). BPD severity was not different between the treatment groups. There were no differences between the groups in the need for mechanical ventilation (22% vs 23%; P = .89), duration of mechanical ventilation (9.7 vs 8.4 days; P = .27), or safety outcomes including severe intracranial hemorrhage (3.4% vs 6.2%, P = .68).
Conclusions: We found that iNO delivered noninvasively to premature infants who have not progressed to early respiratory failure is a safe treatment, but does not decrease the incidence or severity of BPD, reduce the need for mechanical ventilation, or alter the clinical course.
Trial registration: ClinicalTrials.gov NCT00955487.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Comment in
-
Inhaled nitric oxide to prevent bronchopulmonary dysplasia in preterm infants--less than a silver bullet.J Pediatr. 2014 Dec;165(6):1079-81. doi: 10.1016/j.jpeds.2014.08.040. Epub 2014 Sep 27. J Pediatr. 2014. PMID: 25266344 No abstract available.
References
-
- The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med. 1997;336:597–604. - PubMed
-
- Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342:469–74. - PubMed
-
- Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006 Apr 29;:367, 1421–31. - PubMed
-
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. - PubMed
-
- Finer NN, Carlo WA, Duara S, Fanaroff AA, Donovan EF, Wright LL, et al. Delivery room continuous positive airway pressure/positive end-expiratory pressure in extremely low birth weight infants: a feasibility trial. Pediatrics. 2004;114:651–657. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical