ADAM9 is a novel product of polymorphonuclear neutrophils: regulation of expression and contributions to extracellular matrix protein degradation during acute lung injury
- PMID: 25063875
- PMCID: PMC4134955
- DOI: 10.4049/jimmunol.1303370
ADAM9 is a novel product of polymorphonuclear neutrophils: regulation of expression and contributions to extracellular matrix protein degradation during acute lung injury
Abstract
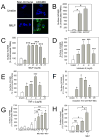
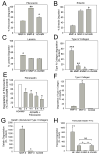
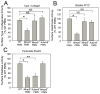
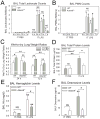
A disintegrin and a metalloproteinase domain (ADAM) 9 is known to be expressed by monocytes and macrophages. In this study, we report that ADAM9 is also a product of human and murine polymorphonuclear neutrophils (PMNs). ADAM9 is not synthesized de novo by circulating PMNs. Rather, ADAM9 protein is stored in the gelatinase and specific granules and the secretory vesicles of human PMNs. Unstimulated PMNs express minimal quantities of surface ADAM9, but activation of PMNs with degranulating agonists rapidly (within 15 min) increases PMN surface ADAM9 levels. Human PMNs produce small quantities of soluble forms of ADAM9. Surprisingly, ADAM9 degrades several extracellular matrix (ECM) proteins, including fibronectin, entactin, laminin, and insoluble elastin, as potently as matrix metalloproteinase-9. However, ADAM9 does not degrade types I, III, or IV collagen or denatured collagens in vitro. To determine whether Adam9 regulates PMN recruitment or ECM protein turnover during inflammatory responses, we compared wild-type and Adam9(-/-) mice in bacterial LPS- and bleomycin-mediated acute lung injury (ALI). Adam9 lung levels increase 10-fold during LPS-mediated ALI in wild-type mice (due to increases in leukocyte-derived Adam9), but Adam9 does not regulate lung PMN (or macrophage) counts during ALI. Adam9 increases mortality, promotes lung injury, reduces lung compliance, and increases degradation of lung elastin during LPS- and/or bleomycin-mediated ALI. Adam9 does not regulate collagen accumulation in the bleomycin-treated lung. Thus, ADAM9 is expressed in an inducible fashion on PMN surfaces where it degrades some ECM proteins, and it promotes alveolar-capillary barrier injury during ALI in mice.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures



References
-
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. - PubMed
-
- Namba K, Nishio M, Mori K, Miyamoto N, Tsurudome M, Ito M, Kawano M, Uchida A, Ito Y. Involvement of ADAM9 in multinucleated giant cell formation of blood monocytes. Cell Immunol. 2001;213:104–113. - PubMed
-
- Izumi Y, Hirata M, Hasuwa H, Iwamoto R, Umata T, Miyado K, Tamai Y, Kurisaki T, Sehara-Fujisawa A, Ohno S, Mekada E. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 1998;17:7260–7272. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

