Phosphodiesterase 2A is a major negative regulator of iNOS expression in lipopolysaccharide-treated mouse alveolar macrophages
- PMID: 25063878
- PMCID: PMC4197568
- DOI: 10.1189/jlb.3A0314-152R
Phosphodiesterase 2A is a major negative regulator of iNOS expression in lipopolysaccharide-treated mouse alveolar macrophages
Abstract
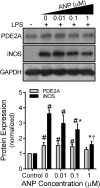
PDE2A is a dual-function PDE that is stimulated by cGMP to hydrolyze cAMP preferentially. In a two-hit model of ALI, we found previously that PDE2A decreased lung cAMP, up-regulated lung iNOS, and exacerbated ALI. Recent data suggest that macrophage iNOS expression contributes to ALI but later, promotes lung-injury resolution. However, macrophage iNOS is increased by cAMP, suggesting that PDE2A could negatively regulate macrophage iNOS expression. To test this, we examined the effects of manipulating PDE2A expression and function on LPS-induced iNOS expression in a mouse AM cell line (MH-S) and primary mouse AMs. In MH-S cells, LPS (100 ng/ml) increased PDE2A expression by 15% at 15 min and 50% at 6 h before decreasing at 24 h and 48 h. iNOS expression appeared at 6 h and remained increased 48 h post-LPS. Compared with control Ad, Ad.PDE2A-shRNA enhanced LPS-induced iNOS expression further by fourfold, an effect mimicked by the PDE2A inhibitor BAY 60-7550. Adenoviral PDE2A overexpression or treatment with ANP decreased LPS-induced iNOS expression. ANP-induced inhibition of iNOS was lost by knocking down PDE2A and was not mimicked by 8-pCPT-cGMP, a cGMP analog that does not stimulate PDE2A activity. Finally, we found that in primary AMs from LPS-treated mice, PDE2A knockdown also increased iNOS expression, consistent with the MH-S cell data. We conclude that increased AM PDE2A is an important negative regulator of macrophage iNOS expression.
Keywords: acute lung injury; atrial natriuretic peptide; cyclic nucleotide; nitric oxide; pneumonia.
© 2014 Society for Leukocyte Biology.
Figures






References
-
- Warner R. L., Paine R., III, Christensen P. J., Marletta M. A., Richards M. K., Wilcoxen S. E., Ward P. A. (1995) Lung sources and cytokine requirements for in vivo expression of inducible nitric oxide synthase. Am. J. Respir. Cell Mol. Biol. 12, 649–661. - PubMed
-
- Kobzik L., Bredt D. S., Lowenstein C. J., Drazen J., Gaston B., Sugarbaker D., Stamler J. S. (1993) Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am. J. Respir. Cell Mol. Biol. 9, 371–377. - PubMed
-
- Kleinert H., Schwarz P. M., Forstermann U. (2003) Regulation of the expression of inducible nitric oxide synthase. Biol. Chem. 384, 1343–1364. - PubMed
-
- Galea E., Feinstein D. L. (1999) Regulation of the expression of the inflammatory nitric oxide synthase (NOS2) by cyclic AMP. FASEB J. 13, 2125–2137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

