An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action
- PMID: 25064128
- PMCID: PMC4268501
- DOI: 10.1038/nm.3599
An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action
Abstract
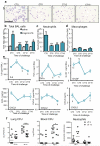
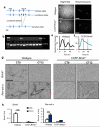
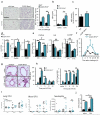
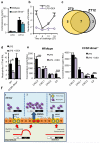
The circadian system is an important regulator of immune function. Human inflammatory lung diseases frequently show time-of-day variation in symptom severity and lung function, but the mechanisms and cell types underlying these effects remain unclear. We show that pulmonary antibacterial responses are modulated by a circadian clock within epithelial club (Clara) cells. These drive circadian neutrophil recruitment to the lung via the chemokine CXCL5. Genetic ablation of the clock gene Bmal1 (also called Arntl or MOP3) in bronchiolar cells disrupts rhythmic Cxcl5 expression, resulting in exaggerated inflammatory responses to lipopolysaccharide and an impaired host response to Streptococcus pneumoniae infection. Adrenalectomy blocks rhythmic inflammatory responses and the circadian regulation of CXCL5, suggesting a key role for the adrenal axis in driving CXCL5 expression and pulmonary neutrophil recruitment. Glucocorticoid receptor occupancy at the Cxcl5 locus shows circadian oscillations, but this is disrupted in mice with bronchiole-specific ablation of Bmal1, leading to enhanced CXCL5 expression despite normal corticosteroid secretion. The therapeutic effects of the synthetic glucocorticoid dexamethasone depend on intact clock function in the airway. We now define a regulatory mechanism that links the circadian clock and glucocorticoid hormones to control both time-of-day variation and the magnitude of pulmonary inflammation and responses to bacterial infection.
Figures


Comment in
-
A local circadian clock calls time on lung inflammation.Nat Med. 2014 Aug;20(8):809-11. doi: 10.1038/nm.3649. Nat Med. 2014. PMID: 25100521
-
Mucosal immunology: killing time in the lungs.Nat Rev Immunol. 2014 Sep;14(9):582. doi: 10.1038/nri3732. Epub 2014 Aug 18. Nat Rev Immunol. 2014. PMID: 25132094 No abstract available.
References
-
- Martinez FJ, Donohue JF, Rennard SI. The future of chronic obstructive pulmonary disease treatment–difficulties of and barriers to drug development. Lancet. 2011;378:1027–1037. Medline CrossRef</jrn>. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 DK59820/DK/NIDDK NIH HHS/United States
- MR/L010240/1/MRC_/Medical Research Council/United Kingdom
- R01 HL105834/HL/NHLBI NIH HHS/United States
- G0802752/MRC_/Medical Research Council/United Kingdom
- 082979/WT_/Wellcome Trust/United Kingdom
- 093612/WT_/Wellcome Trust/United Kingdom
- 1R01AI099479/AI/NIAID NIH HHS/United States
- BB/K003119/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 1R01HL105834/HL/NHLBI NIH HHS/United States
- R01 AI099479/AI/NIAID NIH HHS/United States
- BB/K003097/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/L00254X/1/MRC_/Medical Research Council/United Kingdom
- P01 DK059820/DK/NIDDK NIH HHS/United States
- BB/D004357/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

