HPV prevalence and genotype distribution in a population-based split-sample study of well-screened women using CLART HPV2 human papillomavirus genotype microarray system
- PMID: 25064473
- PMCID: PMC4122758
- DOI: 10.1186/1471-2334-14-413
HPV prevalence and genotype distribution in a population-based split-sample study of well-screened women using CLART HPV2 human papillomavirus genotype microarray system
Abstract
Background: Human papillomavirus (HPV) genotyping assays are becoming increasingly attractive for use in mass screening, as they offer a possibility to integrate HPV screening with HPV vaccine monitoring, thereby generating a synergy between the two main modes of cervical cancer prevention. The Genomica CLART HPV2 assay is a semi-automated PCR-based microarray assay detecting 35 high-risk and low-risk HPV genotypes. However, few reports have described this assay in cervical screening. An aim of the present study, Horizon, was to assess the prevalence of high-risk HPV infections in Copenhagen, Denmark, an area with a high background risk of cervical cancer where women aged 23-65 years are targeted for organized screening.
Methods: Material from 5,068 SurePath samples of women participating in routine screening and clinical follow-up of cervical abnormalities was tested using liquid based cytology, CLART HPV2 and Hybrid Capture 2 (HC2).
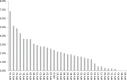
Results: At least one of the 35 defined genotypes was detected by CLART in 1,896 (37%) samples. The most frequent high-risk genotypes were HPV 16 (7%), HPV 52 (5%), and HPV 31 (4%). The most frequent low-risk genotypes were HPV 53 (5%), HPV 61 (4%), and HPV 66 (3%). Among 4,793 women targeted by the screening program (23-65 years), 1,166 (24%) tested positive for one or more of the 13 high-risk genotypes. This proportion decreased from 40% at age 23-29 years to 10% at age 60-65 years. On HC2, 1,035 (20%) samples were positive for any high-risk and thus CLART showed a higher analytical sensitivity for 13 high-risk HPV genotypes than HC2, and this was found in all age-groups and in women normal cytology.
Conclusions: CLART performed well with a positive reproducibility for high-risk genotypes of 86%, and a negative reproducibility of 97%. This report furthermore updates the genotype distribution in Denmark prior to the inclusion of the HPV-vaccinated cohorts into the screening program, and as such represents a valuable baseline for future vaccine impact assessment.
Figures
References
-
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group A review of human carcinogens–Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/S1470-2045(09)70096-8. - DOI - PubMed
-
- Rijkaart DC, Berkhof J, Rozendaal L, van Kemenade FJ, Bulkmans NW, Heideman DA, Kenter GG, Cuzick J, Snijders PJ, Meijer CJ. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78–88. doi: 10.1016/S1470-2045(11)70296-0. - DOI - PubMed
-
- Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Ghiringhello B, Girlando S, Gillio-Tos A, De Marco L, Naldoni C, Pierotti P, Rizzolo R, Schincaglia P, Zorzi M, Zappa M, Segnan N, Cuzick J, New Technologies for Cervical Cancer screening (NTCC) Working Group Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249–257. doi: 10.1016/S1470-2045(09)70360-2. - DOI - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/14/413/prepub
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials