Outcomes of thalassemia patients undergoing hematopoietic stem cell transplantation by using a standard myeloablative versus a novel reduced-toxicity conditioning regimen according to a new risk stratification
- PMID: 25064743
- PMCID: PMC5538782
- DOI: 10.1016/j.bbmt.2014.07.016
Outcomes of thalassemia patients undergoing hematopoietic stem cell transplantation by using a standard myeloablative versus a novel reduced-toxicity conditioning regimen according to a new risk stratification
Abstract
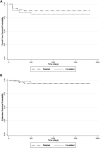
Improving outcomes among class 3 thalassemia patients receiving allogeneic hematopoietic stem cell transplantations (HSCT) remains a challenge. Before HSCT, patients who were ≥ 7 years old and had a liver size ≥ 5 cm constitute what the Center for International Blood and Marrow Transplant Research defined as a very high-risk subset of a conventional high-risk class 3 group (here referred to as class 3 HR). We performed HSCT in 98 patients with related and unrelated donor stem cells. Seventy-six of the patients with age < 10 years received the more conventional myeloablative conditioning (MAC) regimen (cyclophosphamide, busulfan, ± fludarabine); the remaining 22 patients with age ≥ 10 years and hepatomegaly (class 3 HR), and in several instances additional comorbidity problems, underwent HSCT with a novel reduced-toxicity conditioning (RTC) regimen (fludarabine and busulfan). We then compared the outcomes between these 2 groups (MAC versus RTC). Event-free survival (86% versus 90%) and overall survival (95% versus 90%) were not significantly different between the respective groups; however, there was a higher incidence of serious treatment-related complications in the MAC group, and although we experienced 6 graft failures in the MAC group (8%), there were none in the RTC group. Based on these results, we suggest that (1) class 3 HR thalassemia patients can safely receive HSCT with our novel RTC regimen and achieve the same excellent outcome as low/standard-risk thalassemia patients who received the standard MAC regimen, and further, (2) that this novel RTC approach should be tested in the low/standard-risk patient population.
Keywords: Myeloablative; Reduced toxicity; Thalassemia.
Copyright © 2014 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Lucarelli G, Clift R. Marrow transplantation in thalassemia. 3rd. Malden, MA: Blackwell Scientific; 2004. p. 1412.
-
- Lucarelli G, Polchi P, Galimberti M, et al. Marrow transplantation for thalassaemia following busulphan and cyclophosphamide. Lancet. 1985;1:1355–1357. - PubMed
-
- Lucarelli G, Galimberti M, Polchi P, et al. Bone marrow transplantation in patients with thalassemia. New Engl J Med. 1990;322:417–421. - PubMed
-
- Mathews V, George B, Deotare U, et al. A new stratification strategy that identifies a subset of class III patients with an adverse prognosis among children with beta thalassemia major undergoing a matched related allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:889–894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

